Abstract
This study reports the systematic development of cyclodextrin magnetic biocomposite for the remediation of 4-nitrophenol from wastewater. Magnetic biocomposite was synthesized by co-precipitation of Fe2+ and Fe3+ in the presence of NaOH treated pine-cone followed by cross-linking with cyclodextrin using epichlorohydrin, and achieved by an optimization tool. The experiments were designed and the interaction between the working condition variables (CM-CD mass, MNP mass, time and temperature) on the % iron content and 4-NP adsorption capacity were optimized by response surface methodology approach. The temperature and MNP mass both have positive influences on the % iron content and 4-NP adsorption capacity. Crosslinking of cyclodextrin onto magnetite surface was confirmed by transmission electron microscopy, x-ray diffraction, vibrating scanning magnetometer, Fourier transform infrared spectroscopy and thermal gravimetric analysis). Optimum conditions of MNP-EPI-CD were 2.0 g of CM-β-CD, 0.83 g of MNP at 30 °C for 7.40 h which can remove 15.32 mg/g of 4-nitrophenol.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
4-Nitrophenol (4-NP) is the main cause of water pollution due to human population growth, and industrialization, thus posing a significant threat to the environment [1, 2]. 4-NP is used in textiles, tanneries, pharmaceuticals, and pesticides, as such heavily present in drinking and groundwater [3]. 4-NP is highly carcinogenic even at low concentrations, threatening human and animal health as well as the environment [4, 5], as such removing it from wastewater becomes essential.
Photocatalysis, advanced oxidation, membrane filtration, precipitation, and adsorption are some of the methods that have been developed to remove 4-NP from the aquatic environment [1, 6]. Due to its high efficiency, ease of handling, effectiveness in regeneration, and availability of a wide range of adsorbents with desirable physicochemical properties, adsorption is considered the most effective method for removing pollutants from aqueous solution [7]. For 4-NP removal from water and wastewater, numerous adsorbents, including zeolites [8], activated carbon [9], and char ash [10], have been investigated. However, these adsorbents have relatively low adsorption efficiencies, long diffusion paths, and irregular adsorption sites. Nanoscience has made it easier to use nano-magnetic materials like magnetite (Fe3O4), which are very promising adsorbents, that find extensive applications in energy and other environment remediation technologies [11,12,13].
To overcome its non-selective nature, increase its stability, and prevent oxidation, the application of magnetite (Fe3O4) for wastewater remediation necessitates the necessary modifications. Therefore, a coating of β-cyclodextrin (β-CD) which possess hydroxyl functional groups, is effective at modifying the surface of magnetite nanoparticles and providing as an additional adsorption site to remove pollutants, thus addressing Fe3O4 challenges [14]. However, magnetite embedded inside β-CD thus makes it hydrophobic, thus improving its engineering application in wastewater treatment and facilitate separation [14, 15].
The exploration of the response surface methodology (RSM) for the preparation of magnetite pine cone-modified-cyclodextrin (MNP-EPI-CD) adsorbent by means of a physicochemical activation method has not previously been investigated, which is critically influenced by the magnetite-coated biomass amount and reaction temperature in adsorptive removal of 4-NP. Recently, our group has employed (RSM), to optimize and model wastewater treatment processes [12, 16, 17]. This study thus employed a central composite design (CCD) to investigate the influence of the input experimental parameters (MNP mass, β-cyclodextrin mass, temperature, and time) in the preparation of the MNP-EPI-CD adsorbent, on the two response parameters of the 4-NP adsorption capacity, and percentage (%) iron content.
Experimental
The magnetite pine cone (MNP) was prepared according to previously reported studies [11] via alkaline precipitation, as further discussed in the supplementary information (S1). A detailed description of CM-CD polymer [18] is also presented in the supplementary information (S2). For MNP-EPI-CD adsorbents, a variable amount of MNP (0.5–2.0 g), as presented in the design table (Table S1) using central composite design (CCD) via design expert software [19], and a known amount of CM-CD-EPI polymer (0.5–2.0 g), were dissolved in 40 ml of ultrapure water with vigorous stirring at a speed of 1200 rpm. The reaction was heated for different reaction times (3–24 h) at variable temperatures (30–90 °C) under constant stirring, in an inert environment. The resulting nanoparticles were washed with ultrapure water several times to remove any unreacted chemicals and dried in a vacuum oven overnight. The two responses of the study comprise of % Fe content and 4-NP adsorption capacity (mg/g), as presented in Table S2, with 24 randomized runs including 6 replicates. Table S1 indicates the lower and higher limits of every factor. The data were processed using the analysis of variance (ANOVA) and visualized using three-dimensional (3D) surface contour plots.
For each reaction run, the percentage Iron (Fe) content for the MNP-EPI-CD adsorbents was measure with a Rigaku NEX-QC model X-ray fluorescence (XRF) machine, using magnetite analysis application mode. Moreover, the adsorption experiment for 4-NP removal was carried out by adding 0.1 g of adsorbents into 100 ml of 4-NP solution (100 mg/L) inside a 250 mL conical flask, and the mixture was stirred at 145 rpm in an orbital shaker for 2 h. At regular intervals, samples were taken and centrifuged for adsorbate, which was then evaluated using a Perkin-Elmer (USA) Lambda UV–Visible spectrophotometer at 460 nm wavelength. The amount of 4-NP adsorbed was obtained and calculated using Eq. (1) below:
where Ce and Co (mg/L) are the equilibrium and initial concentrations of 4-NP solution, respectively, m (g) is the mass of the adsorbent, and V (L) is the volume of the solution. Solution pH was varied between 2 and 9 for effect of pH. The optimized adsorbent from the response surface model was characterized by XRD, TEM VSM, FTIR and TGA analysis based on previous studies [11, 12].
Results and discussion
Development of regression model equation and statistical analysis
From Table S2, % Iron content for the MNP-EPI-CD adsorbents was observed to range from 50.8 to 92.7%, whilst the adsorption capacity was observed to range from 10.31 to 21.12 mg/g. The sequential square model sum indicates that the models were chosen based on higher-order polynomials and scaled additional parameters. In addition, this selection procedure resulted in the models being rendered non-aliased.
For the response parameters: % Iron content, and 4-NP adsorption capacity, the quadratic model was recommended. The final empirical models in terms of coded factors after exclusion of the insignificant terms from the multiple regression analysis of the experimental data for % Iron content, and 4-NP adsorption capacity using the CCD are presented below:
The A, B, C, D, AB, BD, CD, A2, B2, C2 and D2 have great relevance in explaining the individual and interaction effect of experimental variables on % iron content, according to Eq. 2 above. For the 4-NP adsorption capacity, the A, B, D, AC, BD, CD, A2, B2, C2 and D2 were significant factors based on expression in Eq. 3. In the expression above, positive sign of the coefficient indicates that a synergistic influence on the response, while the negative sign indicates an antagonistic effect on the response.
The results of the analysis of variance (ANOVA) are presented in Table S3, which thus show that Eqs. 2 and 3 accurately represent the true relationship between each response and the significant experimental variables. The ANOVA findings for the quadratic model for the % iron content, and 4-NP adsorption capacity showed model F-values of 570.35 and 235 suggesting that the two models were significant. The F-values and probability-values showed that the second-order effect of temperature (C2) greatly influenced % iron in the MNP-EPI-CD adsorbent. The 4-NP adsorption capacity was observed to be greatly influenced by the second order effect of MNP mass (A2), in line with F-values and probability values.
The correlation coefficient (R2) provides a measure of the model’s variability in the observed response values. The range value of the regression coefficient (R2 = 0.9744–0.9981) for both responses indicates that high correlation between observed and predicted values. Adjusted R2 range value from 0.9506 to 0.9964 confirmed that the model was well fitted. The actual and predicted removal efficiency plot for % iron content, and 4-NP adsorption by MNP-EPI-CD adsorbent are presented in Fig. 1A and B.
Fig. 2A–C depicts the 3D surface plots showing the effects of the MNP-EPI-CD adsorbent experimental parameters on the % iron content. According to Fig. 2A, increasing the reaction temperature resulted to an increase in the % Iron content present in the adsorbent up to a maximum at both short and long reaction times. However, further temperature increases resulted in a decrease in the % Iron present in the adsorbent. On the other hand, at both low and high temperatures, an increase in reaction time resulted in a slight decrease in the % Iron present up to a minimum value, which was followed by a gradual increase in the % Iron at long reaction time. The contour plot has an elliptical line, indicating that the % Iron in the adsorbent is strongly influenced by temperature and time. The reduction in % iron content of MNP-EPI-CD with an increment or reduction in temperature was ascribed to increase in cross-linking between MNP and β-CD-EPI polymers [19].
The 3D response and contour diagrams of the interaction of the experimental inputs on the 4-NP adsorption capacity are presented in Fig. 3A–C. Figure 3B shows interaction effect of reaction temperature and MNP mass while keeping CM-β-CD mass and time fixed at 1.25 g and 1 h, respectively. The 3D plot showed that 4-NP adsorption capacity increased to a maximum value with an increase in MNP mass but decreased slightly with an increase in MNP amount at both low and high reaction temperatures [20]. On the other hand, an increase in temperature led to an increase in 4-NP adsorption capacity at both low and high MNP masses. The plot’s elliptical shape indicates that MNP mass and temperature interact strongly.
The results of the optimization carried out by the RMS methodology are shown in Table S4. As can be seen, the mass of CM-β-CD was 2.0 g, 0.83 g of MNP at reaction temperature of 30 °C for 7.40 h yielded 47.55% iron content of the MNP-EPI-CD adsorbent remove 15.32 mg/g of 4-NP from aqueous solution.
Adsorbent characterization
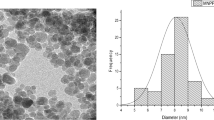
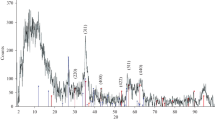
The optimized MNP-EPI-CD, and MNP adsorbents were further subject to XRD, TEM, VSM, FTIR and TGA analysis and adsorption study for comparison. The XRD pattern, TEM image, size distribution and VSM results for MNP and MNP-EPI-CD are shown in Fig. 4A–F. The pinecone coated MNP nanoparticle (Fig. 4A) shows the crystalline structure of the cellulose I peak, located at 2 theta 22.84°, as well as five distinct peaks, located at 30.09°, 35.42°, 43.05°, 56.94°, and 62.51° corresponding to the crystalline structures of magnetite (JCPDS 19-0629) [21]. The MNP-EPI-CD adsorbent had diffraction peaks at 30.2°, 35.7°, 43.2°, 53.6°, 57.2°, 62.8°, and 74.4° attributed to Fe3O4. Due to the presence of pinecone and cyclodextrin, two crystalline structures of cellulose I were also observed at 12.39° and 22.73° for MNP-EPI-CD. The XRD patterns of MNP-EPI-CD reveal that the resulting nanocomposites were crystalline and did not undergo any phase change because of crosslinking.
The VSM technique and magnetization measurements were carried out by measuring the applied field dependence of magnetization cycled between − 15 and 15 kOe at room temperature to comprehend the magnetic properties of MNP-EPI-CD adsorbent. Magnetic saturation of MNP was observed to be 37.50 emu/g which slightly reduced to 21.75 emu/g on crosslinking magnetite pinecone to cyclodextrin (Fig. 4B). Both curves pass through the origin, with low coercivity values of 10.64 and 10.05 for MNP and MNP-EPI-CD, respectively, indicating that the adsorbents exhibit superparamagnetic behaviour. Although the magnetization slightly reduced upon crosslinking, both adsorbents can be easily and rapidly separated by an external magnetic field after the adsorption process. Similar outcomes were reported by Saeb et al. [22] in the synthesis of functionalized magnetite with β-CD hydroxyl-rich precursor. The TEM image of pinecone coated MNP shows the spherical shape with size distribution of 8.23 nm (Fig. 4C and E). The image depicts the spherical shape with a slight increase in mean diameter to 9.77 nm upon cross-linking MNP and CD (Fig. 4D and F). The addition of cyclodextrin and epichlorohydrin may be the cause of the larger nanocomposite size following crosslinking. Cyclodextrin was successfully cross-linked onto the surface of the pinecone coated MNP and cross-linking did not significantly alter the nanoparticle shape.
To confirm the crosslinking of cyclodextrin onto pinecone coated MNP using epichlorohydrin, FTIR analysis was performed and the results are presented in supplementary Fig S1. In the pine cone coated MNP spectrum, the broad stretching vibration at 3307, 2888 and 1622 cm−1 are due to the O–H, C–H and C–O groups, respectively. The band at 1027 and 560 cm−1 are attributed to the antisymmetric glycosidic C–O–C and Fe–O stretching vibration of magnetite. All significant peaks of CM-β-CD and MNP were present in the spectrum of MNP-EPI-CD with a shift and increase in the intensities of some peaks. The broad OH stretching vibration and C–H groups were observed to have increased in intensity and shifted to 3343 and 2883 cm−1, respectively, The C–O peak also slightly increased in intensity with a small shift to 1636 cm−1 which is characterized as the COOM group due the reaction between the COOH group of the CM-β-CD with the suraface of OH groups of magnetite resulting in the formation of iron carboxylate [23]. The C–O–C peaks was also found to increase in intensities due to carbonyl groups from epichlorohydrin and cyclodextrin. This confirms the crosslinking of cyclodextrin onto MNP surface which is in accordance with our previous morphological and XRD analysis. This is coherent with results reported by Baddrudoza et al. [24] when synthesizing carboxymethyl-cyclodextrin conjugated magnetic nano-adsorbent. Thermal degradation profile of MNP and MNP-EPI-CD are presented in supplementary Fig.S2. Both adsorbents show weight loss due to loss of adsorbed water molecules below 100 °C. MNP adsorbent showed 3 weight loss stages. The second loss of 30% was observed between 250 and 380 °C, attributed to the decomposition of the cellulose and hemicellulose from pinecone as was also observed by Pholosi et al. [26]. The third weight loss of 15% is observed between 660–820 °C. Weight loss at 670 °C is attributed to the phase transition from Fe3O4 to FeO, and at 786 °C, deoxidation of FeO occurred since the TGA/DTA analysis was achieved under the nitrogen atmosphere [27]. MNP-EPI-CD had 3 stages of weight loss. The second weight loss of 8% followed between 260 and 340 °C due to the degradation of CM-β-CD, hemicelluloses and cellulose. The last weight loss of 10% was due to magnetite decomposition shifted to temperature between 800 and 900 °C. Crosslinking cyclodextrin onto magnetite surface by epichlorohydrin enhanced the thermal stability of the MNP. The total weight loss of MNP was 45% while that of MNP-EPI-CD was 18%.
Adsorption experiments
Effect of solution pH on 4-NP adsorption
Supplementary Fig. S3 shows the effect of solution pH on the adsorption of 4-NP on MNP and MNP-EPI-CD at the pH ranging from 2 to 9. The adsorption of 4-NP from an aqueous solution was found to be significantly influenced by the pH of the solution. The amount of 4-NP adsorbed on both MNP and MNP-EPI-CD were observed to increase as solution pH increases from pH 2 to pH 3 and reduced as pH is further increased from pH 3 to 9. This could be explained by looking at the 4-NP pka value (7.2) and the pHpzc of the adsorbent materials. pHpzc values of MNP and MNP-EPI-CD were 5.86 and 4.00, respectively and optimum solution pH values for both adsorbents are seen to be below 4-NP pKa value. 4-NP exist as molecules/ non-ionized form at low solution pH, while the adsorbent surface groups are protonated. Higher 4-nitrophenol removal of MNP and MNP-EPI-CD at pH 3 (pH lower than pHpzc for both MNP and MNP-EPI-CD) may have occurred through 2 mechanisms; hydrophobic interaction between pine cone in the MNP bio-composite and 4-nitrophenol and hydrogen bonding between weakly acidic Fe–OH in the MNP as pH is adjusted to 3. Similar results were observed by Zulkurnai et al. [25] when removing 4-NP by Sea Mango based activated carbon.
Comparison of MNP-EPI-CD nanocomposite with other adsorbents
Comparison of maximum adsorption capacities of MNP-EPI-CD and different adsorbents for the removal of 4-NP is shown in Table S5. MNP-EPI-CD is comparable with other adsorbents and therefore, a potential adsorbent for 4-nitrophenol remediation from aqueous solution.
Conclusion
A water soluble cyclodextrin magnetite pine cone (MNP-EPI-CD) adsorbent was successfully prepared and the synthesis procedure was well optimized via response surface methodology in the removal of 4-NP from an aqueous solution. The experimental values were in good agreement with the model predicted values, and the regression analysis. The processing variables were analyzed for optimization, and the results highlighted the positive influence of temperature and MNP mass on the % iron content, and 4-NP adsorption capacity. According to the response surface methodology, the MNP-EPI-CD adsorbent was able to remove 15.32 mg/g of 4-nitrophenol at optimized variables. The MNP-EPI-CD adsorbent demonstrated better performance compared to MNP, in accordance to the characterization analysis, suggesting it could potentially be used for remediation of 4-NP in the ground water.
Data availability
The corresponding author can provide the datasets that were generated/ analyzed during this study upon reasonable request.
References
D. Ewis, M.M. Ba-Abbad, A. Benamor, N. Mahmud, M. Nasser, M. El-Naas, A.W. Mohammad, Int. J. Environ. Res. 16, 23 (2022)
X. Lin, C. Li, Y. He, L. Li, Macromol. Rapid Commun. (2022). https://doi.org/10.1002/marc.202200786
A. Das, A. Dey, J. Environ. Chem. Eng. 8, 103830 (2020)
X. Tian, Y. Liu, W. Chi, Y. Wang, X. Yue, Q. Huang, C. Yu, Water Air Soil Poll. 228, 1 (2017)
R. Sinha, P. Purkayastha, Mater. Lett. 316, 132054 (2022)
D. Ewis, B. Hameed, J. Water Proc. Eng. 41, 102006 (2021)
A. Pholosi, E. Naidoo, A. Ofomaja, Int. J. Environ. Sci. Technol. 16, 6907 (2019)
M. Dogan, F. Temel, M. Tabakci, J. Inorg. Organomet. Polym. Mater. 30, 4191 (2020)
A.I. Ismail, Canad. J. Chem. 93, 1083 (2015)
Y.M. Magdy, H. Altaher, E. ElQada, Appl. Water Sci. 8, 1 (2018)
A. Pholosi, B.E. Naidoo, A.E. Ofomaja, Environ. Sci. Poll. Res. 25, 30348 (2018)
M. Masuku, L. Ouma, S. Sanni, A. Pholosi, Sci. Rep. 12, 18609 (2022)
P. Sareminia, H. Mashhadimoslem, A. Ghaemi, J. Porous Mater. 29, 1853 (2022)
Y. Teng, G. Song, R. Chen, X. Zhang, Y. Sun, H. Wu, B. Liu, Y. Xu, J. Taiwan Inst. Chem. Eng 132, 104153 (2022)
Z.-W. Xie, J.-C. Lin, M.-Y. Xu, H.-Y. Wang, Y.-X. Wu, F.-A. He, H.-L. Jiang, Ind. Eng. Chem. Res. 59, 12270 (2020)
S. Sanni, E. Viljoen, A. Ofomaja, Process Saf. Environ. Prot. 146, 20 (2021)
C.P. Okoli, E.B. Naidoo, A.E. Ofomaja, Environ. Nanotechnol. Monit. Manag. 9, 141 (2018)
M. Fernández, M.L. Villalonga, A. Fragoso, B.N. Caob. Villalong, Process Biochem. 39, 535 (2004).
C.P. Okoli, A.E. Ofomaja, J. Clean Prod. 217, 42 (2019)
J. Dong, L. Shen, S. Shan, W. Liu, Z. Qi, C. Liu, X. Gao, Sci. Total. Env. 806, 151442 (2022)
L. Ouma, A. Pholosi, M. Onani, Phys. Sci; Rev. (2022).
M.R. Saeb, H. Rastin, M. Shabanian, M. Ghaffarid, G. Bahlakehe, Prog. Org. Coat. 110, 172 (2017)
W. Zhang, L. Zhuang, H. Shen, H. Xie, Z. Hu, H. He, J. Sci. Conf. Proc. 1, 211 (2009)
A. Badruddoza, G.S.S. Hazel, K. Hidajat, M. Uddin, Colloids Surf. A: Physicochem. Eng. Asp. 367, 85 (2010)
N.Z. Zulkurnai, N.W.A.Z. Najib, U.F. Md Ali, T. Ru Shien, IOP Conf. Sr. Mater. Sci. Eng. 778, 012154 (2020)
A. Pholosi, S.O. Sanni, S.O. Akpotu, V.E. Pakade, Phys. Sci. Rev. (2023).
M. Mahdavi, M. Bin Ahmad, M.J. Haron, F. Namvar, B. Nadi, M.Z. Ab Rahman, J. Amin. Molec. 18, 7533 (2013)
Acknowledgments
This research was funded by the National Research Foundation (NRF) of South Africa and Vaal University of Technology.
Funding
Open access funding provided by Vaal University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest regarding this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pholosi, A., Sanni, S.O. Synthesis of magnetite (Fe3O4) coated pinecone crosslinked to cyclodextrin and its adsorption behaviour for 4-Nitrophenol: Response surface methodology. MRS Advances 8, 736–742 (2023). https://doi.org/10.1557/s43580-023-00586-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00586-2