Abstract
Dopamine is an efficient building block to produce a versatile coating polymer able to adhere on a vast repertoire of material surfaces. Polydopamine, a dark-bioinspired polymer, is produced by the self-assembly of the dopamine under aerobic conditions in an alkaline environment. The presence of oxygen is crucial for self-polymerization of dopamine in aqueous solution. In this manuscript we show that is possible to drive the polymerization in absence of oxygen exploiting the metabolism of anaerobic photosynthetic purple bacteria.
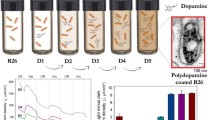
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is the product of the enzymatic decarboxylation of its precursor 3,4-dihydroxyphenylalanine (L-DOPA, Fig. 1a) and is a widespread neurotransmitter in living organisms. The DA structure contains a central benzene ring substitute with two hydroxyl groups in position 1 and 2, forming a catechol which is further substituted with an ethylamine group in position 4. The molecule has a pK = 8.93 and at slightly alkaline pH, and in presence of oxygen or some other oxidant, forms—at room temperature—the dark polydopamine (PDA), a polymer with excellent coating properties. The first report of PDA coatings as simple and versatile strategy for surface modification dates back in 2007 [1] inspired by the composition of adhesive proteins that mussels (Mytilus edulis) secrete to strongly adhere to wet surfaces [2]. The foot proteins in the byssal adhesive plaque of the mussels are rich in L-DOPA and lysine and it has been demonstrated that the strong covalent and noncovalent interactions with substrates is produced by phenolic hydroxyl/quinone groups of DOPA [3]. Furthermore, lysine plays an important role in cross-linking, resulting in solidification of the liquid secreted adhesive protein [3,4,5,6,7].
a Enzymatic decarboxylation of L-DOPA into DA b Self-oxidation of DA to dopamine quinone (DQ) and leucodopaminechrome (DAL). Oxidation of this molecule and subsequent rearrangement resulting in the formation of 5,6-dihydroxyindole (DHI). c Dimers and higher order oligomers dimers self-assemble to form PDA
The mechanism of PDA formation is yet under debate, but it begins with an auto-oxidation reaction [1] of the DA to the dopamine quinone (DQ) via dopamine semiquinone. DQ undergoes a Michael-type intramolecular cycloaddition reaction, forming leucodopaminechrome (DAL) which, in turn, is further oxidized to form the 5,6-dihydroxyindole (DHI, Fig. 1b). The polymerization mechanism of DA (Fig. 1c) is unclear, and nowadays several patterns are equally accepted and competitive: PDA is a covalent polymer and also a supramolecular aggregate of 5,6-dihydroxyindole monomers [8,9,10].
The commonly used protocol for PDA production typically requires alkaline condition (2 mg/mL of commercially available dopamine hydrochloride in 10 mM TRIS buffer at pH 8.5), which ensures the deprotonation of the amino group required for the intramolecular Michael addition [11]. The presence of O2 as an oxidant is crucial for the self-polymerization of DA [12, 13] and the rate of polymerization can be changed by changing the oxygen concentration [14]. The partial removal of O2 is detrimental for the polymerization process slowing down its kinetics. This easy one-step process produces a non-unique and inhomogeneous set of PDA structures and is not yet completely understood; it suffers from low selectivity, is hard to control in situ [15], and depends strongly on the reaction conditions including the reaction time, pH, and temperature. Dopamine polymerization can be also achieved under acidic conditions (pH 4.0) using strong oxidants such as sodium periodate or ammonium persulfate that have a strong impact on PDA film composition and structure [12]. The oxidation can also be obtained by UV irradiation [16] and transition-metal ions that have been shown to catalyse dopamine oxidation in weakly acidic solutions [17].
The structural similarity of PDA with melanin-like materials is relevant in the scientific panorama, specifically for the synthesis of biocompatible adhesive coatings. PDA, thanks to its numerous redox-active quinone and catechol groups, facilitate the extracellular transfer of photosynthetic electrons produced by anoxygenic phototrophic bacteria, a major group of photosynthetic microorganisms widely distributed in Nature [18]. One of the most investigated photosynthetic anoxygenic microorganisms is the purple non-sulphur bacterium Rhodobacter (R.) sphaeroides. The unique feature of photosynthetic organisms to drive their metabolism by capturing solar light [18,19,20] makes this microorganism a model system for several intriguing and sustainable applications spanning from bioremediation of polluted sites [21, 22] to optoelectronics [23,24,25,26,27,28] thanks also to their ability to cope with inorganic nanosized materials such as single-wall carbon nanotubes [31].
The green mutant strains R26 of R. sphaeroides used in this work lacks the carotenoids and their photoprotective role and consequently, this strain is extremely sensitive to oxygen. Here the self-polymerization of DA in both aerobic and anaerobic environment was investigated and compared to the polymerization in presence of the mutant strain, under strictly anoxygenic conditions.
Materials and methods
PDA synthesis
Dopamine hydrochloride (MW = 189.64 uma, CAS 62-31-7) and all chemicals were obtained from Sigma-Aldrich, except for yeast extract obtained from Biolife. All chemicals were used with no further purification. Four dopamine solutions were prepared, dissolving, respectively, 0.1 mM, 0.4 mM, 1 mM and 3.5 mM of the monomer in a growth medium previously optimised for culturing photosynthetic bacteria [29]. The formation of PDA was conducted under aerobic conditions (open vial) and under anaerobic conditions (closed vials previously bubbled with nitrogen).
Experiments were performed in triplicate at increasing concentrations of DA and the formation of PDA was monitored for about 60 h by measuring the absorbance at 535, 588 and 660 nm using a Dr. Lange photometer.
Bacterial growth
Bacterial cells of strain R26 of R. sphaeroides were obtained by the German Collection of Microorganisms and Cell Cultures (DMSZ) and grown in the culture medium [29]. Dopamine hydrochloride (ranging from 0.1 mM to 3.5 mM) was added into the medium under anoxygenic conditions, depleting oxygen by nitrogen insufflation. Biological triplicate of R. sphaeroides R26 was inoculated at a starting concentration of 1 ± 0.5 × 109 CFU/ml (OD535 ~ 0.3) and incubated at room temperature for 4 h in the dark for the consumption of residual oxygen by cellular metabolism. Then, cultures were incubated at 28 °C under steady illumination by quartz halogen lamp (80 W) placed 25 cm away from the vials. Cell densities of the triplicate of unexposed and exposed bacteria at increasing concentrations of DA were monitored for about 30 h by measuring the absorbance at 535, 588 and 660 nm using a Dr. Lange turbidimeter. Heat-inactivated bacteria were used as control.
The autoxidation of dopamine and the consequently polydopamine formation was carried out at room temperature at pH 6.9 in a bacterial growth medium under oxygenic and anoxygenic conditions.
Results
A rapid increase of the optical density (OD) is observed in the first ten hours of the polymerization reaction at 588 nm (Fig. 2a). The initial polymerization rate depends poorly (Fig. 2b) on the initial monomer concentration passing from ~ 0.6 h−1 to ~ 1.5 h−1; this increase is ascribed to the residual oxygen in the bacterial growth medium. Once residual oxygen is consumed, the polymerization cannot proceed further and the OD reaches a plateau. Under oxygenic conditions, the polymerization is sustained much longer, as expected because of the unlimited presence of oxygen, and depends markedly on the initial DA concentration, increasing by a factor of six passing from 0.1 mM to 3.5 mM. A similar trend was registered also when recording the optical density at 535 and 660 nm (Supplementary Fig. S1). The initial polymerization rate acquired after four hours of dark (Fig. 2b) shows that no particular differences were observed between oxygenic and anoxygenic conditions for low concentrations of DA, but the polymerization rate is faster when oxygen is not depleted at higher concentrations, confirming its role as a catalyst in DA oxidation and PDA formation at 1 and 3.5 mM.
a The optical density (OD) of the self-polymerization of DA in aerobic (red dots) and anaerobic (black dots) conditions at [DA] ranging from 0.1 mM to 3.5 mM, over time (in hours). OD was recorded at 588 nm. b Initial polymerization rate of DA in aerobic (circle) and anaerobic conditions (dark circle)
To better investigate the role of oxidative agents in the polymerization process, bacterial cells of the photosynthetic R. sphaeroides 26 were grown under anaerobic conditions in presence of the same four concentrations of DA previously tested. The optical densities of the growing bacterial cultures are shown in Fig. 3 at different DA concentrations as red dots while the negative control experiment showing the growth of the photosynthetic bacterium in plain medium not supplemented with DA, are shown as black squares. Consistently, the OD of the bacterial cultures containing DA are higher than the control, with the sole exception of the lowest DA concentration. In this case, DA exposed bacteria and the control showed similar behaviour. At least at [DA] = 0.1 mM, the monomer does not show any detrimental effect on the bacterial growth. At higher DA concentration, in absence of oxygen, any detrimental effect of the monomer would result in a lower bacterial growth compared to control. The OD would hence result lower in exposed bacteria than in unexposed. Instead, bacterial growth performed at higher DA concentrations show a higher OD than the control, implying the contemporary increase in the biomass and the formation of PDA which absorbs at 588 nm [30]. If oxygen were the sole source of oxidative power for dopamine polymerization, the OD at 588 nm of bacterial growth in presence of DA would be comparable to the control experiment as no polydopamine would form in anoxygenic conditions [12,13,14]. Furthermore, if the monomer DA had any toxic effect, it would limit the anoxygenic bacterial growth, since no or very little PDA would form, and the OD of bacterial growth should be lower than the controls (Fig. 2a).
To confirm that the presence of growing bacteria promotes the polymerization of dopamine, replacing the oxygen role, a further experiment with inactivated R. sphaeroides cells was performed is shown in Fig. 4 where healthy and inactivated bacteria are exposed to dopamine in the polymerization process under anoxygenic conditions. The curve of inactivated R. sphaeroides cells grown in medium supplemented with dopamine and under anoxygenic conditions (empty triangles) follows the same trend of sole dopamine solution (empty dots). A difference in the initial OD is due to the absorption of inactivated cells. As control, the bacterial growth of metabolically active bacterial cells is also represented in Fig. 4.
Conclusions
We have shown a novel, alternative route to the formation of polydopamine in absence of oxygen by exploiting the anoxygenic metabolism of the purple photosynthetic bacteria. Oxygen represents the most relevant oxidant for the formation of this important biocompatible polymer; hence this alternative route may be of interest for the formation of adhesive polydopamine layers in environmental conditions where oxygen is not available. Further comparative experiments with other strains of purple bacteria will help to address some mechanistic details of the polymerization reaction.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
H. Lee, S.M. Dellatore, W.M. Miller, P.B. Messersmith, Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849), 426–430 (2007)
S.H. Ku, J. Ryu, S.K. Hong, H. Lee, C.B. Park, General functionalisation in route for cell adhesion on non-wetting surfaces. Biomaterials 31(9), 2535–2541 (2010)
B.P. Lee, P.B. Messersmith, J.N. Israelachvili, J.H. Waite, Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 41, 99–132 (2011)
H. Seonki, N. Yan Suk, C. Sunghwan, S. In Taek, K. Woo Youn, L. Haeshin, Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 22, 4711–4717 (2012)
M.L. Alfieri, T. Weil, D.Y.W. Ng, V. Ball, Polydopamine at biological interfaces. Adv. Colloid Interface Sci. 305, 102689 (2022)
Y. Liu, K. Ai, L. Lu, Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedicals fields. Chem. Rev. 114(9), 5057–5115 (2014)
L. Rajamani, M. Srinivasan, C.P. Choo, Oxidative polymerization of dopamine: a high-definition multifunctional coatings for electrospun nanofibers—An overview, in Dopamine. ed. by Y. Sarat Chandra (IntechOpen, Rijeka, 2018), p.6
P. Delparastan, K.G. Malollari, H. Lee, P.B. Messersmith, Direct evidence for the polymeric nature of polydopamine. Angew. Chem. Int. Ed. 131, 1089–1094 (2019)
S. Hong, Y.S. Na, S. Choi, I.T. Song, W.Y. Kim, H. Lee, Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 22(22), 4711–4717 (2012)
S. Hong, Y. Wang, S.Y. Park, H. Lee, H. Lee, Progressive fuzzy cation-π assembly of biological catecholamines. Sci. Adv. 4, 7457 (2018)
J. Yang, M.A.C. Stuart, M. Kamperman, Jack of All Trades: Versatile Catechol Crosslinking Mechanisms. Chem. Soc. Rev. 43, 8271–8298 (2014)
Q. Wei, F. Zhang, J. Li, B. Li, C. Zhao, Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem 1, 1430–1433 (2010)
W. Zheng, H. Fan, L. Wang, Z. Jin, Oxidative self-polymerization of dopamine in an acidic environment. Langmuir 31, 11671–11677 (2015)
H.W. Kim, B.D. McCloskey, T.H. Choi, C. Lee, M.J. Kim, B.D. Freeman, H.B. Park, Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interfaces 5(2), 233–238 (2013)
J.H. Ryu, P.B. Messersmith, H. Lee, “Polydopamine Surface Chemistry: A Decade of Discovery” ACS Appl. Mater. Interfaces 10, 7523–7540 (2018)
X. Du, L. Li, J. Li, C. Yang, N. Frenkel, A. Welle, S. Heissler, A. Nefedov, M. Grunze, P.A. Levkin, UV-triggered dopamine polymerization: control of polymerization, surface coating, and photopatterning. Adv. Mater. 26, 8029–8033 (2014)
M. Salomäki, T. Ouvinen, L. Marttila, H. Kivelä, J. Leiro, E. Mäkilä, J. Lukkari, Polydopamine nanoparticles prepared using redox-active transition metals. J. Phys. Chem. B 123(11), 2513–2524 (2019)
G. Buscemi, D. Vona, P. Stufano, R. Labarile, P. Cosma, A. Agostiano, M. Trotta, G. Farinola, M. Grattieri, (2022) Bio-inspired redox-adhesive polydopamine matrix for intact bacteria biohybrid photoanodes. ACS Appl. Mater. Interfaces. 14(23), 26631–26641 (2022)
A. Operamolla, R. Ragni, F. Milano, R.R. Tangorra, A. Antonucci, A. Agostiano, M. Trotta, G.M. Farinola, Garnishing the photosynthetic bacterial reaction center for bioelectronics. Journal of Mater. Chem. C 3(25), 6471–6474 (2015)
F. Milano, M. Trotta, M. Dorogi, B. Fischer, L. Giotta, A. Agostiano, P. Marόti, L. Kálmán, L. Nagy, Light-induced transmembrane proton gradient in artificial lipid vesicles reconstituted with photosynthetic reaction centers. J. Bioenerg. Biomembr. 44(3), 373–384 (2012)
Labarile, R., Farinola, G.M., Varsalona, M., Italiano, F., Buscemi, G., Trotta, M., Grattieri, M. Halotolerance of Rhodobacter sphaeroides for saline and hypersaline wastewater bioremediation, IEEE, 37–42, (2021)
Varsalona, M., Belviso, B.D., Labarile, R., Italiano, F., Farinola, G.M., Trotta, M., Caliandro, R. The role of cytochrome c in the chromate reduction by Rhodobacter sphaeroides IEEE, 96–100, (2022)
F. Milano, A. Punzi, R. Ragni, M. Trotta, G.M. Farinola, Photonics and optoelectronics with bacteria: making materials from photosynthetic microorganisms. Adv. Funct. Mater 29, 1805521 (2019)
G. Buscemi, D. Vona, P. Stufano, R. Labarile, P. Cosma, A. Agostiano, M. Trotta, G.M. Farinola, M. Grattieri, Bio-inspired redox-adhesive polydopamine matrix for intact bacteria biohybrid photoanodes. ACS Appl. Mater. Interfaces 14(23), 26631–26641 (2022)
S. La Gatta, F. Milano, G.M. Farinola, A. Agostiano, M. Di Donato, A. Lapini, P. Foggi, M. Trotta, R. Ragni, A highly efficient heptamethine cyanine antenna for photosynthetic reaction center: from chemical design to ultrafast energy transfer investigation of the hybrid system. Biochim. et Biophys. Acta-Bioenerg 4, 350–359 (2019)
S.R. Liu, L.F. Cai, L.Y. Wang, X.F. Yi, Y.J. Peng, N. He, X. Wu, Y.P. Wang, Polydopamine coating on individual cells for enhanced extracellular electron transfer. Chem. Commun 55, 10535–10538 (2019)
L.D. de Torquato, G. Moura, M. Grattieri, Photobioelectrochemistry of intact photosynthetic bacteria: advances and future outlook. Curr. Opin. Electrochem. 34, 101018 (2022)
J.H. Kim, M. Lee, C.B. Park, Polydopamine as a biomimetic electron gate for artificial photosynthesis. Angew. Chem. 126(25), 6482–6486 (2014)
A. Buccolieri, F. Italiano, A. Dell’Atti, G. Buccolieri, L. Giotta, A. Agostiano, F. Milano, M. Trotta, Testing the photosynthetic bacterium Rhodobacter sphaeroides as heavy metal removal tool. Anal. Chim. 96, 195–203 (2006)
C. Nieto, M.A. Vega, G. Marcelo, E.M. Martín del Valle, Polydopamine nanoparticles kill cancer cells. RSC Adv. 8, 36201 (2018)
A. Antonucci, M. Reggente, C. Roullier, A.J. Gillen, N. Schuergers, V. Zubkovs, B.P. Lambert, M. Mouhib, E. Carata, L. Dini, A.A. Boghossian, Carbon nanotubes uptake in cyanobacteria for near-infrared imaging and enhanced bioelectricity generation in living photovoltaincs. Nat. Nanotechnol. 17, 1111–1119 (2022)
Acknowledgments
The authors wish to acknowledge Ms. Vittoria Lorusso for drawing the purple bacterium in the graphical abstract.
Funding
This work was funded by the Fonds National Suisse de la Recherche Scientifique, project Phosbury—Photosynthetic bacteria in Self-assembled Biocompatible coatings for the transduction of energy—(Project Nr CRSII5_205925/1) and by the project Mission Innovation POA 2021–2023 Italian Energy Materials Acceleration Platform—IEMAP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Labarile, R., Varsalona, M., Vona, D. et al. A novel route for anoxygenic polymerization of dopamine via purple photosynthetic bacteria metabolism. MRS Advances 8, 423–428 (2023). https://doi.org/10.1557/s43580-023-00566-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00566-6