Abstract
Aluminium-doped zinc oxide (AZO) is a potential low-cost alternative to indium tin oxide (ITO) for application in optoelectronic devices as a transparent conducting thin film. Typically, AZO thin films are deposited using expensive, high vacuum equipment with high energy cost and materials wastage. In this study, slot-die coating was used as an inexpensive alternative to vacuum deposition to form AZO nanoparticle thin films under ambient laboratory conditions. The films were characterised structurally, optically and electrically and compared with a commercially obtained AZO film fabricated using physical vapour deposition (PVD). Structural characterisation of the nanoparticle film shows uniform coverage across the substrate with increased crystal quality following annealing in Ar up to 500 \(^{\circ }\)C. The optical properties of the nanoparticle film exhibit a wider band gap than the PVD film, while the high density of grain boundary defects between the nanoparticles inhibits sheet conductivity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Slot-die coating, illustrated in figure 1, belongs to a class of methods known as pre-metered or curtain coating where the liquid coated film thickness can be precisely controlled based on the flow rate of the liquid through a die and the speed which the substrate moves through the liquid curtain [1]. Once evaporated, the residue on the substrate forms a dry film, which can be used for low-cost optoelectronic devices.
Conventional vacuum deposition methods such as chemical vapour deposition (CVD) and physical vapour deposition (PVD), are inefficient in terms of material usage and require a large amount of energy to operate [2].
For example, during a 5-hour magnetron sputter deposition of a 2.9 \(\mathrm \upmu\)m thick TiN film, the coating process alone requires 84 kWh, more than three quarters of the 112 kWh total energy consumed. For CVD fabrication of TiCN/Al\(_2\)O\(_3\), the heating process for an 18.5 h, 10 \(\mathrm \upmu\)m film is 437 kWh, which accounts for almost half of the 974 kWh total energy. In terms of material processing, 0.436 g/s of PVD material makes it to the substrate while 0.964 g/s is wasted onto the carousel and chamber walls (\(\sim 30 \%\) efficient). For CVD, the coating forms at \(\mathrm 5.26\times 10^{-3}\) g/s, however, \(\mathrm 2.08\times 10^{-2}\) g/s is wasted in the chamber and sample holder (\(\sim 20 \%\) efficient).
In comparison, rapid manufacturing via slot-die coating wastes significantly less material. For example, 4.3 \(\mathrm \upmu\)L of the nanoparticle ink used in this study is required to produce one 100 nm thick film coated on a 25 \(\times\) 75 mm substrate. From a syringe of 5 mL, only 3 \(\mathrm \upmu\)L is wasted between the syringe and slot-die, achieving near 100% material usage efficiency. Further, the slot-die machine used in this work requires just 0.02 kWh, thereby requiring significantly less energy as substrates can be coated in a matter of seconds.
For 2D electrical devices such as sensors, photovoltaics (PV) and displays, transparent conducting oxides (TCO) are useful for charge carrier collection with minimum optical interference. Currently, ITO is favoured in the TCO market due to its low resistivity (\(\mathrm \sim 10^{-4}\) \(\Omega \textrm{cm}\)) and excellent transmittance (\(\mathrm \sim 85\%\)), however, due to the high cost of indium, it is important to identify an optoelectronically similar material with lower cost [4].
ZnO is a popular alternative to ITO due to its excellent optical transmittance (\(\mathrm \sim 80\) \(\mathrm \%\)) and wide band gap (\(\mathrm E_g = 3.3\) eV), however, with a higher resistivity than ITO (\(\mathrm \sim 1-100\) \(\Omega \mathrm cm\)), improvements can be made by the incorporation of either Al, Ga or In. Of these, Al and Ga are favourable [5, 6]. The incorporation of Al into the Zn lattice sites provides some unique properties such as an increased band gap due to the occupation of states in the conduction band from the substitution of \(\mathrm Al^{3+}\) to the \(\mathrm Zn^{2+}\) sites. This apparent blue-shift is known as the Moss–Burstein effect [7].
Since the radius of the \(\mathrm Al^{3+}\) ion (0.053 nm) is smaller than the \(\mathrm Zn^{2+}\) ion (0.074 nm), the substitution of Al in the Zn sites forms a shorter covalent bond length causing the crystallite size to reduce [8,9,10]. This improves chemical stability in reducing environments and stabilises the electrical properties as the aluminium dopant provides free charge carriers and impedance to the chemisorption of surface oxygen.
Methods
For slot-die deposition of AZO thin films, AZO nanoparticles dispersed in isopropyl alcohol (IPA) were purchased from Merck and used directly. These were compared to PVD AZO thin films obtained commercially from Delta Technologies Ltd.
Substrates used for slot-die coating
25 mm \(\times\) 75 mm \(\times\) 1 mm soda lime glass substrates were used for slot-die deposition of AZO nanoparticles. A 25 mm \(\times\) 75 mm \(\times\) 0.7 mm ITO substrate was also used for electrical characterisation and as a reference for the optical performance of the nanoparticle film. This ITO substrate consists of a \(\sim\)25 nm ITO sputtered conductive film.
Slot-die coating parametrisation
The characteristics of slot-die processed dry films are governed by coating parameters that are based on the physical properties of the ink. A calculator was designed based on slot-die theory to set these parameters [11]. The Merck 901092 25 ml ink used is a 2.5 wt% dispersion of AZO nanoparticles in 2-propanol solvent. The dynamic viscosity (\(\mu\)) was measured as 2.1 \(\mathrm \upmu Pa\cdot s\) and the surface tension (\(\mathrm \sigma\)) was 25 mN/m. The liquid density (\(\mathrm \rho\)) was measured as 0.806 g/ml. Using an Ossila goniometer, the dynamic contact angle on glass (\(\mathrm \theta\)) was measured as \(\mathrm 13\) \(^\circ\). The slot-die coater was set to \(V=20\) mm/s coating speed and \(R=5.67\) \(\upmu\)L/s ink injection flow rate with slot width \(W=100\) \(\upmu\)m. H is the gap height between the slot-die head and substrate, and was set at 219 \(\upmu\)m to produce a \(100 \pm 35\) nm thick dry film in a single pass.
Post-deposition treatment
Fifteen samples were made using slot-die deposition and then annealed for 1, 2 and 3 h at 150 \(^\circ\)C, 250 \(^\circ\)C, 350 \(^\circ\)C, 450 \(^\circ\)C, and 500 \(^\circ\)C each in a 50 \(\upmu\)L/s flow of Ar inert gas.
Characterisation measurements
The structural, optical and electrical measurements included scanning electron microscopy (SEM) using a Tescan Mira3 to determine the sample morphology and thickness, grazing incidence x-ray diffraction (GIXRD) using a Rigaku SmartLab SE (Cu K-\(\alpha\) source) in parallel beam (PB) mode to identify crystal phase and size, and UV–Vis spectroscopy with a Shimadzu UV-2600 double beam spectrophotometer with a bare glass reference, to deduce the optical band gap.
A 4-point probe with 1 mm spacing was used with an injection current ranging from −50 to +50 \(\upmu\)A to measure sheet resistance. Hall effect was used with a 10 mA injection current and 4490 G magnetic field to measure carrier mobility. The measurement was calibrated using a Wheatstone bridge with known resistance.
Results and discussion
Structure and morphology
SEM images in figure 2, show the surface morphologies and cross-sections of the as-deposited nanoparticles and commercially obtained PVD films. Uniform thickness and substrate coverage was achieved with the slot-die deposition, as shown in figures 2 (a) and (b). The thickness achieved was 70 nm, which is within the range estimated using slot-die theory [11]. The nanoparticle average size is 15 nm.
For comparison, PVD films are shown in figures 2 (c) and (d). These images indicate that the PVD film is also of uniform thickness across the substrate but composed of a granular morphology with grain sizes ranging from 50 to 250 nm and thickness of 1 \(\upmu\)m. This larger grain size and thickness is attributed to prolonged deposition time and higher temperature [12].
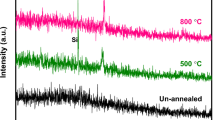
The crystal structure and phase of both the slot-die and PVD films are illustrated in the XRD reflection patterns in figure 3(a). For all samples measured, the peak positions match closely with a ZnO reference indicating wurtzite phase structure. As expected, the amplitude and full width at half maximum of the peaks from the annealed nanoparticle films are lower and wider, respectively, compared with the PVD film, indicating smaller crystallite sizes. However, figure 3(b) also shows that the nanoparticle film quality could be improved by annealing in an Ar atmosphere up to 500 \(^\circ\)C. This was supported by determining the that crystallite size increased linearly from 6 nm to 9 nm and at 500 \(^\circ\)C, the crystallite size achieved is similar to that of the PVD film as determined from the Scherrer equation [13]:
where \(\tau\) is the crystallite size, K=0.9 is a constant, \(\lambda = 0.15406\) nm (Cu K-\(\alpha\) X-ray source), \(\beta\) is the width of the peak at half maximum, and \(\theta\) is the Bragg angle of the peak.
The improvement in the crystallite size from 6 nm to 9 nm with annealing temperature can be explained as atoms in energetically unfavourable sites are able to diffuse at higher temperatures, allowing them to move to an energetically more favourable site within the lattice [14].
Optical properties
UV–Vis spectroscopy was used to deduce the optical band gap of the nanoparticle and PVD films with an ITO film to reference. A blank soda-lime glass slide was used for calibration. From the Bouguer-Beer-Lambert law, the absorption coefficient \(\alpha\), is calculated using [15]:
where d is optical path length and;
where \(\lambda\) is wavelength, \(T(\lambda )\) is the intensity of transmitted light, \(I(\lambda )\) is the intensity of the incident light and \(R(\lambda )\) is the intensity of reflected light. Since only 4% of incident light is reflected, \(A_{abs}\) is approximated to depend only on \(T(\lambda )\). The optical band gap is then deduced using equations 2 and 3, with the Tauc equation [16]:
where h is Planck’s constant, \(\nu\) is the photon frequency, B is a constant related to band edge tailing and \(E_g\) is the optical band gap. For direct band gaps \(\gamma = \frac{1}{2}\) which is constant for this type of exciton transition.
The optical data in figure 4(a), shows that the slot-die nanoparticle film exhibits a band gap at 4.37 eV which is significantly blue-shifted relative to the PVD film at 3.58 eV and the ITO film at 4.17 eV. Closer inspection of the spectrum for the slot-die film in figure 4(b) shows a feature close to the PVD film curve with a band gap of 3.27 eV, close to bulk ZnO at 3.3 eV [17].
There are two possible mechanisms which account for the physical nature of the slot-die film spectrum: the Burstein–Moss (BM) effect and quantum confinement. In the case of BM, non-uniform distribution of Al in the nanoparticles will result in a distribution of optical band gaps. Quantum confinement effect in ZnO is achieved when the particle size is below 6 nm [18] and is identified by correlating the calculated Brus radius with particle sizes measured in the SEM images [19].
The average particle size can be calculated from the absorption edge using the Brus equation [18, 19]:
where \(\Delta E(R)\) is the blue-shifted band gap, \(E_g(R)\) is the bulk band gap, h is Plank’s constant, \(\epsilon = 3.85\), \(\epsilon _0 = 8.0\), R is the confinement radius and \(m_e^* = 0.28 m_o\) and \(m_h^* = 0.59 m_o\) [7] are the electron and hole effective masses. This gives a particle size of \(3.3\pm 0.5\) nm, significantly smaller than the sizes measured using SEM (\(\sim\)15 nm) and XRD (9 nm).
For BM, the carrier concentration, n, can be estimated using [20]:
where \(\Delta E_{BM}\) is the optical band gap shift due to Moss-Burstein effect. This gives a carrier concentration of \(n=6.7\times 10^{20}\) for the nanoparticle film and \(n=8.6 \times 10^{19}\) for the PVD film, showing a factor of 7 difference between the two samples. Comparing this with electrical measurements will confirm if this blue-shift is due to BM effect.
Electrical properties
In the nanoparticle film, grain boundary defects inhibit carrier mobility such that the resistivity large. This can be deduced accurately by measuring the cross-section resistance.
An ITO substrate provides a medium with known resistivity which can be used as a reference. The nanoparticle layer acts as a series resistor where the injected current passes directly to the ITO substrate.
The resistivity for ITO was measured first as \(\rho = 1.44 \times 10^{-4}\) \(\Omega \mathrm cm\). The AZO nanoparticle film resistivity is calculated using:
where r is the radius of the probe used to inject the current to the film. The AZO nanoparticle resistivity is therefore \(\rho = 4.15\times 10^{-2}\) \(\Omega\)cm. Comparing to the PVD film with \(\rho = 7.22\times 10^{-4}\) \(\Omega\)cm, the large number of grain boundary defects in causes a significantly higher resistivity (\(\sim 60\times\) increase).
The carrier concentration, n, which depends on current density, J, and drift velocity, u, can be deduced from the Hall effect measurement according to the equation:
where q is the carrier charge. The ITO carrier concentration is measured first as \(n= 1.14\times 10^{21}\) \(\mathrm cm^{-3}\). The combined AZO nanoparticle film on ITO sample has a carrier concentration of \(n=1.96\times 10^{20}\) \(\mathrm cm^{-3}\). The reduced carrier concentration is attributed to an increase in drift velocity as charge carriers move between layers. Using this, the AZO nanoparticle carrier concentration is deduced as \(n = 2.1 \times 10^{21}\) \(\mathrm cm^{-3}\). Compared to the PVD AZO at \(n = 3\times 10^{20}\) the larger carrier concentration may be attributed to intrinsic doping as previously identified with the UV–Vis measurements in figure 4 as the factor of 7 difference between the samples is also observed in these measurements. The increase in n from the UV–Vis measurement, is attributed to non-parabolicity of the ZnO band structure, which is determined using Kane’s dispersion [21].
Conclusion
Slot-die coating was used to rapidly fabricate relatively low-cost AZO nanoparticle thin films on glass substrates. Relative to vacuum deposition methods, this technique resulted in uniform thin films with little waste and low-energy budget. Annealing the nanoparticle thin films in inert gas was observed to increase the crystallinity of the slot-die films and the crystallite size became comparable to that of the PVD film. Optical measurements indicated a significant blueshift of the AZO band gap for the nanoparticle thin film relative to the PVD film, caused by the Burstein-Moss effect from impurity doping. Electrical measurements indicated that the AZO nanoparticle films do not conduct laterally as a result of the high density of grain boundary defects. The properties of the AZO nanoparticle films suggest that they may be suitable for large scale manufacturing of devices for optical applications but not yet electronic. Future work will address this, for example by studying the potential for optical (UV) curing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
M.S. Carvalho, H.S. Kheshgi, Low-flow limit in slot coating: theory and experiments. AIChE J. 46(10), 1907–1917 (2000). https://doi.org/10.1002/aic.690461003
M. Gassner, M. Rebelo de Figueiredo, N. Schalk, R. Franz, C. Weiß, H. Rudigier, C. Mitterer, Energy consumption and material fluxes in hard coating deposition processes. Surf. Coatings Technol. 299, 49–55 (2016). https://doi.org/10.1016/j.surfcoat.2016.04.062
A. Baptista, F. Silva, J. Porteiro, J. Míguez, G. Pinto, Sputtering physical vapour deposition (PVD) coatings: a critical review on process improvement and market trend demands. Coatings (2018). https://doi.org/10.3390/COATINGS8110402
S. Hartner, M. Ali, C. Schulz, M. Winterer, H. Wiggers, Electrical properties of aluminum-doped zinc oxide (AZO) nanoparticles synthesized by chemical vapor synthesis. Nanotechnology (2009). https://doi.org/10.1088/0957-4484/20/44/445701
Z. Zhang, C. Bao, S. Ma, S. Hou, Effect of crystallinity of ZnO buffer layer on the properties of epitaxial (ZnO:Al)/(ZnO:Ga) bi-layer films deposited on c-sapphire substrate. Appl. Surf. Sci. 257(17), 7893–7899 (2011). https://doi.org/10.1016/j.apsusc.2011.04.070
M. Gabás, P. Torelli, N.T. Barrett, M. Sacchi, J.R. Ramos Barrado, Electronic structure of Al- and Ga-doped ZnO films studied by hard X-ray photoelectron spectroscopy. APL Mater. 10(1063/1), 4863595 (2014)
B.E. Sernelius, K. Berggren, C.G. Granqvist, Band-gap tailoring of ZnO by means of heavy Al doping. Phys. Rev. (1988). https://doi.org/10.1103/PhysRevB.37.10244
M.M. Rahman, M.K.R. Khan, M.R. Islam, M.A. Halim, M. Shahjahan, M.A. Hakim, J.U. Khan, Effect of Al doping on structural, electrical, optical and photoluminescence properties of nano-structural ZnO thin films. J. Mater. Sci. Technol. 28(4), 329–335 (2012). https://doi.org/10.1016/S1005-0302(12)60064-4
T.H. Chen, T.C. Cheng, Z.R. Hu, The electrical and optical properties of AZO thin film under different post-annealing temperatures. Microsyst. Technol. 19(11), 1787–1790 (2013). https://doi.org/10.1007/s00542-013-1837-5
S. Suwanboon, P. Amornpitoksuk, A. Haidoux, J.C. Tedenac, Structural and optical properties of undoped and aluminium doped zinc oxide nanoparticles via precipitation method at low temperature. J. Alloys Compd. 462(1–2), 335–339 (2008). https://doi.org/10.1016/j.jallcom.2007.08.048
X. Ding, J. Liu, T.A.L. Harris, A review of the operating limits in slot-die coating processes. AIChE J. 62, 2508–2524 (2016). https://doi.org/10.1002/aic.15268
Hamelmann, F. U, Thin film zinc oxide deposited by CVD and PVD. J Phys Conf Ser. 764, 012001 (2016). https://doi.org/10.1088/1742-6596/764/1/012001
L. Alexander, H.P. Klug, Determination of crystallite size with the x-ray spectrometer. J. Appl. Phys. 21(2), 137–142 (1950). https://doi.org/10.1063/1.1699612
J. Sengupta, R.K. Sahoo, K.K. Bardhan, C.D. Mukherjee, Influence of annealing temperature on the structural, topographical and optical properties of sol - gel derived ZnO thin films. Mater. Lett. 65, 2572–2574 (2011). https://doi.org/10.1016/j.matlet.2011.06.021
I. Mills, T. Cvitas, K. Homann, N. Kallay, K. Kuchitsu, IUPAC green book. (2007). https://doi.org/10.1039/9781847557889
P. Makuła, M. Pacia, W. Macyk, How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J. Phys. Chem. Lett. 9(23), 6814–6817 (2018). https://doi.org/10.1021/acs.jpclett.8b02892
A. Janotti, C.G. Van De Walle, Fundamentals of zinc oxide as a semiconductor. Rep. Progress Phys. (2009). https://doi.org/10.1088/0034-4885/72/12/126501
N.S. Pesika, K.J. Stebe, P.C. Searson, Relationship between absorbance spectra and particle size distributions for quantum-sized nanocrystals. J. Phys. Chem. B 107(38), 10412–10415 (2003). https://doi.org/10.1021/jp0303218
L. Brus, Electronic wave functions in semiconductor clusters: experiment and theory. J. Phys. Chem. 90(12), 2555–2560 (1986). https://doi.org/10.1021/j100403a003
T.S. Moss, The interpretation of the properties of indium antimonide. Proc. Phys. Soc. B 67(10), 775–782 (1954). https://doi.org/10.1088/0370-1301/67/10/306
W.M. Kim, I.H. Kim, J.H. Ko, B. Cheong, T.S. Lee, K.S. Lee, D. Kim, Density-of-state effective mass and non-parabolicity parameter of impurity doped ZnO thin films. J. Phys.D: Appl. Phys. (2008). https://doi.org/10.1088/0022-3727/41/19/195409
Acknowledgments
We acknowledge ReNu (EPSRC: EP/S023836/1) and PV Paint (EPSRC: EP/T005491/1) for support in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matheson, E.D., Xu, X., Qu, Y. et al. A structural, optical and electrical comparison between physical vapour deposition and slot-die deposition of Al:ZnO (AZO). MRS Advances 8, 330–335 (2023). https://doi.org/10.1557/s43580-023-00554-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00554-w