Abstract
The temperature phase diagram stability and physical properties of a binary Silver–Aluminium (Al–Ag) alloy system have been calculated using Thermo-Calc 2021b software at a temperature range of 250–1300 K. Six single-solid phases other than the liquid phase, elemental Ag (fcc_Ag), elemental Al (fcc_Al), hcp_A3, cub_A13, and bcc_Ag were obtained from the equilibrium calculation. Ten regions of the double phase were obtained and evaluated. Three intermediate phases were also obtained from the phase diagram with five invariant reactions, namely one eutectic reaction (at 840.00 K), two peritectic reactions (at 1051.22 K and 1003.18 K), and two peritectoid reactions (at 730.58 K and 881.40 K) were equally found. Sixteen regions other than elemental Ag and Al at 0, and 100, respectively, were obtained. Maximum solid solubility obtained was 5.60 Al mass percent at 730. 58 K for Al in Ag and 58.17 Ag mass percent at 840.00 K for Ag in Al. From the temperature–composition phase diagram, the melting point for pure silver and aluminium were equally inferred as 1234.96 K and 933.50 K, respectively, which agrees with experimental standards. These results play a significant role in the processing of Al–Ag alloy for different industrial-related applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phases are the fundamental building blocks of material systems [1, 2] and can be used to predict stable phases as a function of temperature and composition [3]. There are several factors that contribute to the cost difference between simulation model-based approaches and traditional experimental methods when predicting the phase properties of materials. One key advantage of simulation-based approaches, such as those found in software, like Thermo-Calc and Fact-Sage, is their ability to accurately predict the behaviour of materials using established thermodynamic models, without the need for expensive and time-consuming experimental trials. These models allow researchers to focus specifically on the area of research interest for the proposed alloy, eliminating the need for extraneous experimentation that may be required with traditional methods. This not only reduces costs and saves time but also enables researchers to make more informed decisions about the properties and behaviour of materials, leading to more accurate and effective results. This will ultimately ensure that the new alloy being designed meets the specific property requirements in terms of composition, processing, and microstructural expectations.
Aluminium-based alloys are complex materials and have been found to possess many advantages, including low density, increased strength (depending on the alloy), good resistance to corrosion, and excellent thermal and electrical conductivity, amongst others [2, 4, 5]. The Al–Ag alloy is a binary system whose phase diagram has been analysed by several researchers using different experimental techniques [6,7,8]. Unfortunately, these works are old with a lot of discrepancies in the results reported. The Al–Ag alloy has been found suitable for grain refining and hardening [9], as electrical conductors [10], and formation of screen printing pastes [11]. The relatively high cost of obtaining silver has also further hindered continued research into this binary alloy system [10]. Optimizing the processing parameters, such as temperature and composition, to prevent defects and achieve desired properties is crucial when preparing alloys. This study addresses this need by utilizing Thermo-Calc software to simulate and predict the phase equilibria of the Al–Ag binary alloy under various conditions, providing researchers with valuable information to improve their experimental approach. By reducing the time and resources required for trial-and-error experiments, this study can significantly improve the preparation method for alloys, specifically for the Al–Ag binary alloy.
Methodology
For this study, Thermo-Calc 2021b software was used to calculate the thermodynamic properties and obtain the phase diagram for the binary Al–Ag system. The CALculation of PHAse Diagrams (CALPHAD) method was used to calculate thermodynamic properties of material systems [1, 12] and the binary calculation template adopted for the equilibrium phase diagram of the Al–Ag binary system. This is consistent with methodologies used by other researchers for previous related works [6,7,8, 13, 14]. The calculation was performed at a temperature range of 250 K to 1300 K and a pressure of 1 bar. The TCBIN: TC Binary Solutions v1.1 database was used to perform the thermodynamic calculation for phase stability of the Al–Ag alloy binary system.
Results and discussion
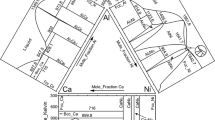
Figure 1 shows the result of the Thermo-Calc phase equilibria calculation for Al–Ag binary alloy and that obtained from previous research [7, 15,16,17], which demonstrate a satisfactory level of conformity. The Al–Ag phase diagram of Fig. 1a has a total of sixteen fields, including elemental Ag at 0 and elemental Al at 100. Phase properties analysis shows six single phases, namely the liquid phase, fcc_Ag, cub_A13, hcp_A3, bcc_A2, and fcc_Al phases. When compared with Fig. 1b, the phases are labelled as \((Ag),\) μ, \(\delta\), \(\beta\), and \((Al)\), respectively. From the six single phases identified from Fig. 1, three intermediate phases are also observed, namely cub_A13 μ, bcc_A2 \((\beta )\), and hcp_A3 \((\delta )\). Most research studies on Al–Ag alloy have always been on the metastable hcp_AgAl intermediate phase [6, 8, 15, 18]. The ten double-phase reactions obtained are listed in Table 1.
Location of the phase boundaries of the Al–Ag alloy binary system is another aspect where authors’ discrepancies existed for the different results presented which lead McAlister [7] to conclude that there is need to further study these boundary structures. Figure 1a shows a boundary structure for the Ag-rich side extending from 0 to 40.31 at% Al at temperature range of 300.00 K to 1234.96 K. Another boundary structure for the Al-rich side is observed to extend from 40.31 to 99.93 at% Al at a temperature range of 300.00 K to 840.01 K. These results were consistent with the metallographically assessed experimental results of [19,20,21] for both the Al- and Ag-rich sides. Other notable boundaries seen in Fig. 1 include the \(\delta\) boundary present at both the Ag-rich and Al-rich solidus terminals, the μ boundary which is only limited to the Ag-rich terminal, and the \(\beta\) boundary.
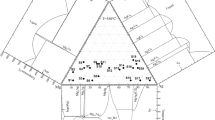
Figure 2 shows the intermediate phases, namely the metastable \(\delta\), \(\beta\), and μ intermediate phases. The hcp_A3 intermediate phase from Fig. 2a stabilizes below 734.47 °C compared to 727 °C reported by [7]. This phase extends from 23.02 at% Al at 608.74 °C to 41.27 at% Al at eutectic temperature 566.85 °C. This relatively agrees with 23.0 at% Al at 611 °C to 41.9 at% Al at 567 °C assessed by [7]. The \(bcc\_A2\) intermediate phase (Fig. 2a) stabilizes above 606.94 °C compared to 603 °C reported by [7]. The bcc \(\beta\) intermediate phase extends from 20.90 at% Al at 776.27 °C to 27.86 at% Al at 730.08 °C. This is significantly lower to 20.5 at% Al at 779 °C to 29.8 at% Al at 727 °C assessed by [7]. A cubic μ intermediate phase (Fig. 2b) stabilizes below 457.43 °C and extends from 19.32 at% Al to 27.16 at% Al. This is higher than the single-phase field of 21.2–24.3 at% Al at 300 °C assessed by [7].
Enlarged portion of Fig. 1a showing the a intermediate and b cubic intermediate phases
The hexagonal phase in Al–Ag alloys (\(\delta\)) has been reported to have a composition near Ag2Al with a probable suggestion that Ag atoms diffuse to stacking faults in the fcc Al-rich alloys [22]. This has thus lead to a debate on the composition and temperature needed to predict the direct transition from the \(\delta\) phase to the \(\beta\) phase with series of discrepancies between experimental and calculated results reported at the intermediate phases [13]. Spencer et al. [23] predicted that the transition to occur between 0.1 and 0.2 at% Al around 1075–1125 K. Hume-Rothery et al. [20] using detailed X-ray and microscopic analysis reported the phase transformation between 24.6 and 25.8 at% Al at a temperature below 604 °C. In this study (Fig. 2), a direct transition from the hcp \((\delta )\) intermediate precipitation phase to high-temperature \(\beta\) phase is predicted to occur at 23.94 at% Al and 606.90 °C which agrees with [20].
Five invariant reactions, namely one eutectic, two peritectic, and two peritectoid regions, are observed in the binary phase diagrams of Fig. 1. The eutectic reaction (Eq. 1) occurred at 566.85 °C at eutectic composition ratio of 65.57 at% Al to 34.43 at% Ag and is consistent with the generally acceptable value of 567 °C [7, 9, 10] but slightly differs at the eutectic composition of 67 at% Al reported by [21, 22] using wet chemical analysis.
The \(\beta\) intermediate phase occur at 778.07 °C and 20.79 at% Al from a peritectic reaction (Eq. 2) which agrees with the 778 °C and 20.50 at% Al obtained by [7] albeit to a small degree of uncertainty.
A second peritectic reaction (Eq. 3) for \(\delta\) metastable intermediate phase also occurs at 32.76 at% Al and 730.03 °C and is consistent with the value of 726 ± 3 °C at 32.7 at% Al reported [7] to about 4.03 °C uncertainty.
A cubic μ structure intermediate phase is formed by a peritectoid reaction (Eq. 4) at 457.43 °C and 22.25 at% Al. A second peritectoid reaction (Eq. 5) is formed at the metastable \(\delta\) intermediate phase at 608.25 °C and 23.05 at% Al. The peritectoid temperatures are both a little higher and lower than that obtained by Ref. [7] at 450 ± 3 °C and 22.9 at% Al and 610 ± 3 °C and 23.5 at% Al, respectively.
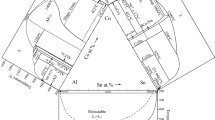
The melting point temperature of pure Ag (fcc_A1) shown in Fig. 3a occurs at 961.81 °C and agrees with the widely accepted value of 961.8 °C [7, 24]. Figure 3b also predicts the melting point temperature of pure Al at 660.35 °C and relatively agrees with widely accepted literature value of 660.45 °C [7, 24]. Purity of the materials affects the melting point values of any experimental procedure.
Enlarged portions of Fig. 1 showing a Ag melting point and b Al melting point
The maximum solid solubility of Al in Ag contained in the Ag-rich terminal (fcc_Ag) obtained is 19.17 at% Al at peritectoid temperature of 457.43 °C as against a higher value of 20.4 at% Al at about 450 °C assessed by [7]. For the Al-rich solid solution (fcc_Al), the maximum solid solubility of Ag in Al obtained is 25.81 at% Ag at eutectic temperature of 566.85 °C which is higher than 23.5 at% Ag at relatively the same eutectic temperature of 567 °C reported by [7].
Conclusion
The temperature–composition phase diagram of the Al–Ag alloy binary system was calculated using the Thermo-Calc (TCBIN database) software. The phase diagram showed in total 16 fields, including the terminal regions at 0 and 100 Al mass percent. Six single phases were equally observed with three intermediate phases, namely μ, \(\beta\), and \(\delta\). Five invariant reactions were equally obtained from the phase diagrams. The melting temperature for pure silver and aluminium inferred from the Thermo-Calc calculation was consistent with widely accepted literature values. The calculation also gave the maximum solubility of Al in Ag and Ag in Al to be 5.60 Al mass percent and 58.17 Ag mass percent, respectively. These results are important for developing novel alloys (particularly suited for thermal applications) from the Al–Ag alloy binary system. The use of the Thermo-Calc simulation model-based software for thermodynamic analysis offers several advantages over traditional methods, including affordability, efficiency, and increased precision. The software can also serve as a guide for experimental work, allowing researchers to focus on specific alloy compositions whilst still obtaining valuable insights.
Data availability
Data used in this study are simulated.
References
Thermo-Calc, The CALPHAD Methodology - Thermo-Calc Software, (2016). https://thermocalc.com/about-us/methodology/the-calphad-methodology/ accessed August 28, 2022).
H.L. Chen, Q. Chen, A. Engström, Development and applications of the TCAL aluminum alloy database. Calphad Comput. Coupling Phase Diagr. Thermochem. 62, 154–171 (2018). https://doi.org/10.1016/J.CALPHAD.2018.05.010
T. Turpin, J. Dulcy, M. Gantois, Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: experimental study and theoretical modeling. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 36, 2751–2760 (2005). https://doi.org/10.1007/s11661-005-0271-4
Azom, Aluminium and aluminium alloys—characteristic advantages and beneficial properties of aluminium extrusions, (2008). Accessed August 28, 2022 from https://www.azom.com/article.aspx?ArticleID=4192.
S. Thapliyal, M. Komarasamy, S. Shukla, L. Zhou, H. Hyer, S. Park, Y. Sohn, R.S. Mishra, An integrated computational materials engineering-anchored closed-loop method for design of aluminum alloys for additive manufacturing. Materialia (2020). https://doi.org/10.1016/J.MTLA.2019.100574
T.H. and T.S. Kozo Osamura, Takao Nakamura, Akiko Kobayashi (1987) Chemical composition of G.P. zones in Al-Ag alloys. Pergamon J. 21: 255–258
A.J. McAlister, The Ag-AI (silver-aluminum) system. Bull. Alloy Phase Diagrams 8, 526–533 (1987)
R. Baur, V. Gerold, The existence of a metastable miscibility gap in aluminium-silver alloys. Acta Metall. 10(6), 637–645 (1962). https://doi.org/10.1016/0001-6160(62)90053-6
AmericanElements, Aluminum Silver Alloy | AMERICAN ELEMENTS ®, (2021). Accessed August 28, 2022 from https://www.americanelements.com/aluminum-silver-alloy.
A. Mamala, T. Knych, P. Kwasniewski, A. Kawecki, G. Kiesiewicz, E. Sieja-Smaga, W. Sciȩzor, M. Gniełczyk, R. Kowal, New Al-Ag alloys for electrical conductors with increased current carrying capacity. Arch. Metall. Mater. 61, 1875–1880 (2016). https://doi.org/10.1515/amm-2016-0302
S. Fritz, M. Konig, S. Riegel, A. Herguth, M. Horteis, G. Hahn, Formation of Ag/Al screen-printing contacts on B emitters. IEEE J. Photovoltaics. 5, 145–151 (2015). https://doi.org/10.1109/JPHOTOV.2014.2364117
S.J. Ikhmayies, Phase analysis of the Si-O2 system (2017), pp. 333–342. https://doi.org/10.1007/978-3-319-52192-3_33.
S.S. Lim, P.L. Rossiter, J.E. Tibballs, Assessment of the AI-Ag binary phase diagram. Calphad 19, 131–141 (1995)
K. Osamura, T. Nakamura, A. Kobayashi, T. Hashizume, T. Sakurai, An AP-FIM study on metastable phases in Al-Ag binary alloy. Acta Metall. 34, 1563–1570 (1986). https://doi.org/10.1016/0001-6160(86)90101-X
N.A. Zarkevich, D.D. Johnson, Predicted hcp Ag-Al metastable phase diagram, equilibrium ground states, and precipitate structure. Rev. B—Condens. Matter Mater. Phys. Phys. (2003). https://doi.org/10.1103/PhysRevB.67.064104
Himikatus, Фaзoвaя диaгpaммa cиcтeмы Ag-Al, (n.d.). Accessed August 31, 2022 from https://himikatus.ru/art/phase-diagr1/Ag-Al.php.
SGTE, Collection of Phase Diagrams: Ag-Al Data from alloy Database 1 bar, (2017). https://www.crct.polymtl.ca/fact/phase_diagram.php?file=Ag-Al.jpg&dir=SGTE2017.
S.W. Fu, C.C. Lee, A study on intermetallic compound formation in Ag–Al system and evaluation of its mechanical properties by micro-indentation. J. Mater. Sci. Mater. Electron. 29, 3985–3991 (2018). https://doi.org/10.1007/s10854-017-8340-1
T.P. Hoar, R.K. Rowntree, A note on the Ag-rich Al-Ag alloys above 600 "C. J. Inst. Met. 45, 119–124 (1931)
W. Hume-Rothery, G.V. Raynor, P.W. Reynolds, H. Packer, The constitution and structure of alloys of intermediate composition in the system Cu-ln, Cu-Al, Cu-Ge, Ag-Al, and Ag-Ge. J. LnsL MeL. 66, 209–239 (1940)
G. Massart, P. Desre, E. Bonnier, Thermodynamics of A1-Ag alloys. J. Chim. Phys. 67, 1485–1488 (1970)
R.B. Nicholson, J. Nutting, The metallography of precipitation in an Al-16% Ag alloy. Acta Metall. 9, 332–343 (1961)
P.J. Spencer, O. Kubaschewski, Monatshefte fiir Chemic A Thermodynamic Evaluation of the Ag-Al System**, 1987.
AmericanElements, Melting point of common metals, alloys and other materials, (2006). Accessed November 10, 2021 from http://www.americanelements.com/amp.meltingpoint.html.
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing personal or financial interests have influenced the research findings herein.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ben, F., Olubambi, P.A. Phase and properties prediction of Al–Ag binary system using thermo-calc. MRS Advances 8, 577–582 (2023). https://doi.org/10.1557/s43580-023-00551-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00551-z