Abstract
The present work analyzes the effect of different Zr contents on the microstructure and thermal behavior of non-irradiated UO2. A set of Zr-doped UO2 (0, 20, 40, 80, and 100 wt%) pellets was prepared via solid-state synthesis by mimicking the chemical bonding between ZrO2 and UO2. After sintering, the Zr-doped UO2 monoliths were characterized by scanning electron microscopy, Raman spectroscopy, and X-ray diffraction. Oxidation of the Zr-doped UO2 samples has been monitored by thermogravimetric analysis. Results of thermal behavior under air and low oxygen partial pressure show a profound effect of delayed oxidation with the addition of Zr to UO2 and then an increased oxidation resistance of UO2 to U3O8 when compared to pure UO2 pellet.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In light water reactors (LWRs), UO2 pellets are mainly used as fuel within a zirconium-based alloy clad to protect the fuel pellets and to avoid radionuclides releasing into the coolant [1]. During in-reactor operation radiation effects, thermal expansion and microstructural alterations, i.e., the production of gaseous elements (mainly Xe and Kr) and Fission Products (FP), induce dimensional changes and swelling of the fuel pellet [2, 3]. Particularly, the thermal gradient at which the nuclear fuel is subjected during operation is one of the reasons of the deformation caused in the pellet, which could affect to its interaction with the cladding. Thus, a potential pellet-cladding contact could take place because of a decrease in the cladding diameter (creep-down due to pressure from the primary coolant) [2] and an increase in the pellet diameter. This phenomenon is known as Pellet–Cladding Interaction (PCI) and occurs in the majority of commercial fuel rods [3].
The contact between pellet and cladding, given through a chemical bonding layer, first occurs at the inter-pellet spaces while a continuing pellet fragmentation (radial and axial cracks) takes place simultaneously. The interaction layer consists of two regions. First region occurs closer to the cladding (polycrystalline ZrO2) and the second layer nearer the fuel pellet is an interphase formed by solid solutions of (U, Zr)O2 and an amorphous phase (induced by radiation). This second layer is composed by variable relative concentrations of U and Zr [2]. Because it may induce cladding fuel failures in commercial LWRs during a power transient, it is considered as a mid-priority phenomenon in the investigation of the safety approach under dry storage [4]. In addition, it is one of the causes identified as leading to potential fuel failure. Most of the related literature with UO2–ZrO2 solid solution system comes from severe accident scenarios such as Fukushima in Japan [5, 6]; because of core melting in Nuclear Power Plants (NPPs), U and Zr could interact.
Moreover, the risk of enhanced PCI grows with burnup. In fact, evidences on Zr–oxide layer formation and Zircaloy-Fuel gap closure were observed in high-burnup fuels (HBU). Studies conducted on fuel with burnups > 55 MWd·kg U−1 showed the formation of a restructured region, named as “rim” structure or HBU structure at the periphery of UO2 pellets [7]. As the burnup increases, some effects turn out to appear in this new region, i.e., smaller grains, lattice contraction (recrystallization), higher porosity, hardness decrease, and the closure of pellet–cladding gap in fuel rods [8].
Understanding the potential chemical oxidation resistance of UO2 (matrix fuel) to U3O8 by ZrO2/Zr system in case of undetected damaged cladding (zirconium alloy), and a potential air intrusion, is relevant in terms of assessing fuel integrity. Therefore, assuming evidences on Zircaloy-Fuel gap closure (Zr–UO2 layer formation from HBU PWR fuels) [9,10,11], here we conduct characterization of Zr-doped UO2 pellets including morphological, chemical, and crystallographic analyses. Furthermore, we present some results based on the oxidation behavior of these samples under dry or transportation conditions that probe the chemical oxidation resistance of UO2 to U3O8, when they form part of the (U, Zr)O2 solution, and then mimicking the chemical bonding between ZrO2 and UO2.
Materials and methods
Preparation of model materials: UO2–ZrO2 samples
Unirradiated Zr-doped UO2 pellets were obtained via solid-state synthesis from ZrO2 and UO2 powders, with an initial composition of the powders 0, 20, 40, 80, and 100 wt% ZrO2. Powdered ZrO2 Puratronic ® 99.978% was provided by Alfa Aesar (monoclinic structure; specific surface area, SSA 6.41 ± 0.03 m2 g−1; particle size distribution, PSD 2.02 µm). Natural UO2 powders were obtained via Ammonium Di-Uranate (ADU) by precipitation in NH4OH followed by denitration/dehydrating, calcination, and thermally annealing in a N2–4.7%H2 atmosphere at 1100ºC to attain UO2 (Cubic fluorite structure; SSA 3.75 ± 0.04 m2 g−1; PSD 3.68 µm). Raman spectroscopy (Fig. 1) was used to confirm the structure of the starting powders. Raman spectrum of UO2 powder (Fig. 1A) shows the well-known features of UO2: (a) the triply degenerate T2g mode at 445 cm−1 (fluorite structure) [12] and (b) the first overtone of the LO mode (2LO) centered at ~ 1140 cm−1 [12,13,14]. The ZrO2 spectrum (Fig. 1B) shows the characteristics described in the literature [15].
Mixtures of Zr and U oxides (including undoped UO2 and ZrO2) were prepared by mechanical dry blending from mixing weighted amounts of UO2 and ZrO2 powders, together with 1 wt% of binder EBS (Ethyl Bis Stearamide, C38H76N2O2, Tokyo Chemical Industry) [16], using low-energy ball milling. Then, powder mixtures were sintered at 1675 °C for 4 h in a reducing atmosphere flow (N2–4.7%H2) [17, 18]. Samples were characterized after being sintered: surface morphology and average grain size calculation by SEM; BET SSA (Specific Surface Area) with N2; the purity and crystalline structure by both XRD and RQPA (Rietveld Quantitative Phase Analysis); Raman spectroscopy (laser λexc = 633 nm) and density by Archimedean immersion.
Oxidation experiments of UO2–ZrO2 pellets
To study the oxidation behavior of the prepared materials, Zr-doped UO2 samples were tested by thermogravimetric analysis (TGA). A TGA Q50 thermobalance (TA Instruments) was used under a synthetic air flow (N2–21%O2) and a special mixture with low oxygen content (N2–1%O2) at a constant rate of 60 mL min−1. The thermal treatment consisted of heating sintered fragments up to 900 ºC with flowing air or 1% O2–N2.
Results and discussion
Characterization of mixed oxides sintered pellets
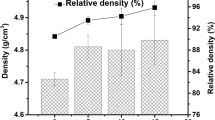
Table 1 shows the characterization results (SSA, lattice parameters, crystalline phase contents, and crystal system) of the as-prepared Zr-doped UO2 (0, 20, 40, 80, and 100 wt%) pellets. The SSAs are approximately 0.3 m2·g−1 and average grain sizes in the range of 4.90 to 7.61 µm. Bulk density determined by Archimedes’ method decreased and relative densities in % (RD, using theoretical densities of 10.97 and 5.68 g cm−3 of UO2 and ZrO2 correspondingly) increased from 9.1 g cm−3 (83%) for UO2 to 8.6 (87%, 20 wt% ZrO2), 8.1 (92%, 40 wt% ZrO2), 6.1 (90%, 80 wt% ZrO2) and 5.5 g cm−3 (97%) for pure ZrO2 as the Zr content, because of dopant effect.
Different crystalline phases are identified by XRD as Zr content increased (Fig. 2A).
In the recorded XRD pattern of pure UO2, the presence of a single cubic phase confirms the purity of the sample, and the (111) peak at low 2θ ~ 28.558° is consistent with the presence of a slightly hyperstoichiometric layer UO2+x on the surface and with a thickness of the order of tens nm [19]. In addition, the diffractograms at doping concentrations of 20 and 40 wt% ZrO2 clearly reveal the apparition of an additional diffraction peak at 2θ ~ 29.8° due to the tetragonal ZrO2 phase, which decreases in the sample doped with 80 wt% ZrO2, once monoclinic lattice appeared, and completely fades in pure ZrO2. This observed peak is in agreement with literature data [15, 20]. Indeed, m-ZrO2 is the thermodynamically stable phase at room conditions [15]. Also, in this sample, an additional peak growth on 2θ ~ 31.5° suggests a non-dissolved fraction of ZrO2 in the UO2 matrix upon sintering or cooling [20]. From the results by RQPA, one might think that a solid solution should be also present in the tetragonal and the monoclinic phase. Anyhow, the solubility of UO2 seems to be greater in the tetragonal phase since it shows higher differences in the lattice parameters than that from the pure ZrO2 phase. All these facts mean that, from the data obtained by XRD, it is possible to get an approach on the solubility limit of Zr in the cubic UO2 matrix in these conditions. The UO2 solid solution was cubic and extended up to 20 wt% ZrO2. Segregation of tetragonal ZrO2 phase is identified even at concentrations below 40 wt% of ZrO2. Far beyond this ZrO2 concentration, the monoclinic phase starts to appear.
Figure 3 shows the microstructure of the polished sintered pellets in backscattered electron (BSE) mode. Normalized Raman spectra recorded on sintered pellets are displayed in Fig. 2B. Pure UO2 spectrum is characterized by the presence of the same features than that described in Fig. 1. The weak bands observed at ~ 570 cm−1 and at ~ 630 cm−1 are assigned to the LO mode (lattice distortion), i.e., structural defects in the cuboctahedral symmetry of the interstitial oxygens, respectively, due to slight oxidation to UO2+x. This is consistent with XRD results, confirming the formation of an oxidized thin layer on the surface of UO2. The peaks identified at ~ 326, 497, and 657 cm−1 are characteristic peaks of m-ZrO2. Considering mixed oxides Raman spectra, the band associated with the T2g phonon at ~ 445 cm−1 (characteristic of the fluorite structure) continues broadening and getting smaller as the ratio U/Zr decreases, indicating a lattice distortion. New bands corresponding to Zr–O stretching (Ag) are observed at ~ 244 cm−1, ~ 497 cm−1, and ~ 636 cm−1 for UO2-80 wt% ZrO2 and ZrO2 samples.
The overall interpretation from all these analysis reveals non-uniform distribution of Zr in the UO2 matrix and ZrO2 segregation in grain boundaries, presumably because the solubility limit is reached in the fabrication procedure.
Oxidation behavior of mixed oxides (U, Zr)O2
Using TGA in air, the thermal oxidation behaviors of (U, Zr)O2 samples containing a range of Zr concentrations are assessed. To understand the effect of Zr on the formation of U3O8, the in situ analysis of the oxidation reaction performed by TGA at different oxygen partial pressure conditions is presented in Fig. 4 for all the studied materials.
Differences in oxidation behavior of the studied (U, Zr)O2 pellets as a function of Zr content (0–100%) and oxygen partial pressure are observed. In general, a delay in the UO2 oxidation is found when increasing the Zr doping concentration. XRD analyses of the oxidized sample (not shown) from pure UO2 confirmed complete conversion to U3O8. When samples were oxidized in air, the two representative oxidation transitions (UO2 → U4O9/U3O7 → U3O8) are observed as a two-step weigh change. However, this behavior is not followed for the sample doped with 80 wt% ZrO2. On the contrary, in 1%O2–N2, the two-step transitions are continuously softened with greater Zr content.
The TGA indicates that, as expected, increasing Zr content on UO2 matrix enhances the stability of the intermediate U oxidation products and slowed the subsequent oxidation rate.
Conclusions
We examined the effects of Zr doping on the thermal stability of UO2 under dry interim storage conditions (air or 1%O2–N2). Mixtures of UO2 and ZrO2 in a range of possible compositions across the layer (PCI phenomenon) are tested to examine the effect of Zr content and oxygen concentration (gas phase) and simulate ZrO2/UO2 bonding (chemical adhesion) in HBU fuels. Systematic oxidation studies on x wt%ZrO2–UO2 (x = 0, 20, 40, 80, and 100) solid samples were analyzed under non-isothermal thermoanalytical conditions (10 ºC min−1, 900 ºC). The thermal analysis indicates hindered of matrix oxidation attributed to the presence of zirconia. The present preliminary results studied as a representative example of a typical HBU fuels and PCI showed that the presence of Zr in UO2 fuel matrix provides oxidation resistance to U3O8.
Therefore, the results of this analysis are important for understanding the behavior of SNF under dry or transportation conditions, once closure of pellet–cladding gap occurs. In order to improve this assumption to real irradiated fuel, further research involving other characterization techniques and considering the occurrence of FP in the Zr/ZrO2 must be explored.
References
IAEA, Thermophysical Properties of Materials for Nuclear Engineering: A Tutorial and Collection of Data (International Atomic Energy Agency, Vienna, 2009)
C. Ciszak et al., J. Nucl. Mater. 520, 110–120 (2019)
D.G. Frost et al., J. Nucl. Mater. 528, 151876 (2020)
OCDE/NEA, Pellet-clad Interaction in Water Reactor Fuels : Seminar Proceedings Aix-en-Provence, France, 9–11 March 2004. Nuclear Science, OCDE, Paris, 2005.
F. Tanabe, J. Nucl. Sci. Technol. 48(8), 1135–1139 (2011)
H. Ding et al., NPJ Mater. Degrad. 6(1), 10 (2022)
K. Lassmann et al., J. Nucl. Mater. 226(1), 1–8 (1995)
(NRC), U.S.N.R.C., Standard Review Plan for Dry Cask Storage Systems Final Report. Washington, 2010.
W.A. Lambertson, M.H. Mueller, J. Am. Ceram. Soc. 36(11), 365–368 (1953)
D.R. Olander, J. Nucl. Mater. 115(2), 271–285 (1983)
R.A. Clark et al., NPJ Mater. Degrad. 4(1), 4 (2020)
J.M. Elorrieta et al., Phys. Chem. Chem. Phys. 18, 28209–28216 (2016)
T. Livneh, E. Sterer, Phys. Rev. B 73(8), 085118 (2006)
H. He, D. Shoesmith, Phys. Chem. Chem. Phys. 12, 8108–8117 (2010)
C. Ciszak et al., J. Raman Spectrosc. 50(3), 425–435 (2019)
J. Auborn, J. Choo, Mechanisms of lubrication in powder metallurgy, in Advances in Powder Metallurgy and Particulate Materials. 1993.
A. Milena-Pérez et al., J. Nucl. Mater. 543, 152581 (2021)
S. Fernandez et al., J. Eur. Ceram. Soc. 36(14), 3505–3512 (2016)
G. Leinders et al., J. Nucl. Mater. 459, 135–142 (2015)
T. Wauters, Investigation of the Lattice Parameter and Sintering Characteristics of Uranium Dioxide Doped with Zirconium and Gadolinia, ed. by M. Verwerft and T. Cardinaels (Campus Groep T, Leuven, Belgium, 2014).
Acknowledgments
The research activities presented were partially supported by the Spanish Ministry of Science and Innovation (Grant PID2021-124913OA-I00 IONMAT project) and the European Joint Programme on Radioactive Waste Management (EURAD) (EU Grant agreement: 847593). The authors acknowledge Sofía Durán for the assistance with instrumentation.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodríguez-Villagra, N., Bonales, L.J., Fernández-Carretero, S. et al. Exploring a surrogate of Pellet–Cladding interaction: Characterization and oxidation behavior. MRS Advances 8, 238–242 (2023). https://doi.org/10.1557/s43580-022-00475-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-022-00475-0