Abstract
Radiation-induced graft polymerization of poly(N-vinylcaprolactam) onto silicone catheters by direct irradiation method was studied. The effects of the irradiation dose, as well as the monomer concentration, on the grafting efficiency were studied. The conditions for achieving maximum grafting yield were observed at 30% of monomer concentration in toluene at 50 kGy. The graft polymerization was examined by different characterization methods, including measurements such as thermogravimetric analysis, infrared, water contact angle, and swelling. The temperature-responsive behavior of smart grafted copolymer was studied by swelling at different temperatures. Differently from pristine silicone catheter, the N-vinylcaprolactam-grafted catheters were able to load vancomycin and sustain the release for 30 h.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary catheters are one of the most widely used medical devices since are assistants for the treatment of various procedures such as surgeries, chemotherapy, and kidney tests.[1,2] These can be intermittent or indwelling, the first is used each time that it is necessary to drain the bladder, while the last is left in the bladder and helps to collect the urine. The employment of indwelling catheters is one of the most common causes of nosocomial infections; about 20% of bacteremia in the intensive care unit and 50% in long-term care centers are due to these devices. In addition, it is estimated that urinary catheters are responsible for between 70 and 80% of urinary infections, known as catheter-associated urinary tract infections (CAUTI). These infections are generally produced by the formation of bacterial biofilms, whose conformation protects bacteria from antimicrobials and host defenses, making them highly resistant.[3]
Latex and silicone urinary catheters are commonly used, due to these minimize urethral trauma. However, silicone catheters are less prone to blockage, making them ideal for indwelling treatments.[4] Silicone is a biocompatible, hypoallergenic synthetic polymer with excellent mechanical properties and chemical resistance, wished characteristics for its application in the manufacture of medical devices.[5,6] The problem with silicone devices is that since it is a hydrophobic material, it suffers from the adhesion of bacteria and the formation of biofilms. To mitigate this problem, antimicrobial coatings have been developed that prevent proliferation, but it continues in research.
One of the ways to provide the surface with antimicrobial properties is through surface modification with stimulus-responsive polymers that allow the loading and release of antimicrobial drugs. Stimulus-responsive polymers are polymeric materials that change their structure in the presence of an external stimulus such as temperature or pH. Poly(N-vinylcaprolactam) (PNVCL) is a biocompatible and thermo-responsive polymer that has a lower critical solution temperature (LCST), that is, its structure is less hydrophilic at temperatures higher than its critical temperature (32–34°C).[7] Due to its critical temperature range, the PNVCL is a promising material for drug delivery under physiological conditions.[8,9] This material has been used for the synthesis of injectable hydrogels to encourage the growth of cartilage tissue, showing promising results in in vitro tests.[10] Furthermore, NVCL can copolymerize to form micelles or hybrid gels with a high potential for drug release.[11,12] For example, Singh et al. synthesized amphiphilic block copolymers of N-acrylamidohexanoic acid and NVCL to form micelles, which allow the encapsulation and delivery of hydrophobic drugs.[13]
Gamma radiation is a high-energy radiation that produces the matter ionization. When a polymer is irradiated, radicals are created along its chain that act as growth points for the graft. The formation of grafts using gamma radiation as an initiator has the advantage of reproducibility, not requiring high temperatures, and avoiding material contamination since it does not need initiators.[14,15] Due to the above, the use of gamma radiation for the development of biomaterials has increased in recent years, reporting materials used for dressing, drug delivery systems, tissues engineering, and biosensors.[16]
This work proposes the modification of silicone catheters using gamma radiation to graft PNVCL, which is a thermo-responsive polymer that gives the catheter the ability to load and release a drug, which may reduce infection incidences in this device. The model drug was vancomycin, an antibiotic against gram-positive bacteria such as Staphylococcus aureus, Streptococcus pyogenes, and Streptococcus pneumoniae. Vancomycin is employed for serious infection treatments and bacteria resistant to other antibiotics, such as methicillin-resistant Staphylococcus aureus.[17,18] S. aureus is one of the bacteria that most cause infection in catheters, and vancomycin is an antibiotic that has high activity against these bacteria.
Materials and methods
Materials
Silicone catheters with a diameter of 5 mm and thickness of 2 mm were obtained from China and washed with ethanol for 8 h and then dried under reduced pressure. N-vinylcaprolactam was purchased from Sigma Aldrich (USA) and distilled under reduced pressure before use. Toluene and methanol were from J.T. Baker (Mexico) and used as received. Distilled water was used for the reaction and analysis. Vancomycin was acquired from Fagron (Colombia) and used as received.
Grafting of SRcat-g-NVCL
Grafting of N-vinylcaprolactam onto silicone catheter, SRcat-g-NVCL was synthesized by direct irradiation method using a 60Co γ-ray source.
The glass ampoules were prepared containing a silicone catheter (2.5 cm length) with a solution of monomer concentration in toluene (from 5 to 50% of NVCL vol.%) or methanol (between 10 and 60% of NVCL vol.%) at different doses and dose rate ≈ 8.5 ± 0.5 kGy/h. Grafting was evaluated by using Eq. 1:
where Wo and Wg are the initial and grafted weight of the films, respectively.
Characterization
For determination of the swelling limit, the catheters were immersed into distilled water at different times, the excess of solution on the catheter’s surface was wiped off with paper, and the swollen catheters were weighed. The swelling yield of the samples was calculated by Eq. 2.
where Ws and Wd are the weights of the swollen and initial films, respectively.
The contact angle was measured at room temperature using a goniometer (DSA 100, Krüss GmbH, Germany) and distilled water. Before the measure, the catheters were flattened until they reached a homogeneous surface and dried. The contact angle was recorded using the computer software at 0 and 5 min. Each measurement was conducted in triplicate.
The lower critical solution temperature (LCST) was evaluated with distilled water swelling measurements at temperatures between 25 and 36°C, where the LCST was defined as the inflection point of the swelling vs. temperature plot (Boltzmann function fitting).
FTIR-ATR (attenuated total reflection) spectra of the starting and modified SR catheters were analyzed using a Perkin-Elmer PARAGON 500 spectrometer (USA), in the horizontal attenuated reflectance mode, and a SeZn glass was used as contact with the sample surface, and 16 scans.
Thermogravimetric analysis (TGA) measurements were carried out on a TAinstruments TGA Q50 (USA) ramping from 25 to 800°C at a heating rate of 10°C/min under a nitrogen atmosphere.
Vancomycin loading and release
Vancomycin loading was performed in grafting catheters with 3, 10, and 18% for PNVCL. The dried catheters were immersed in 3 mL of 2 mg/mL vancomycin solution at 25°C. The amount of load was calculated through UV absorbance measurement at 275 nm employing a calibration curve, Eq. 3 with r2 = 0.999.
Vancomycin release was performed into phosphate buffer at pH 7.4 and 37°C with constant mechanical stirring. The release profiles were monitored by UV spectrometry at 270 nm. The concentration of the released drug was calculated using a calibration curve (Eq. 4, r2 = 0.999). Measurements were conducted in triplicate.
A SPECORD 200 PLUS brand spectrophotometer from Analytikjena (Germany) was used for the measurements.
Results
Grafting PNVCL onto silicone catheters (SRcat-g-PNVCL)
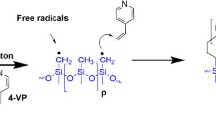
The modification of the silicone catheters was performed using the direct irradiation method, in which the monomer and the matrix to graft are irradiated simultaneously. When silicone comes into contact with radiation, it forms different radicals (Fig. 1(a)), of which radical IV is the most stable.[19,20,21] The variables that interfere in this process are the solvent, the monomer concentration, and the irradiation dose.
(a) Radical formation by silicone irradiation with gamma rays; (b) Grafting as function of monomer concentration in toluene and MeOH, dose 50 kGy, dose rate 8.5 ± 0.1 kGy/h; and (c) Grafting as function of dose at different monomer concentrations 20%, 30%, and 40% in toluene, dose rate 8.5 ± 0.1 kGy/h.
The solvent must allow the interaction between the monomer and the matrix and a total monomer dissolution. Toluene and methanol were tested as solvents; toluene allowed a greater diffusion of the monomer into the matrix, which resulted in an increase in the PNVCL grafting percentage, as shown in Fig. 2(b). Figure 2(b) also shows the behavior of the PNVCL grafting in relation to the monomer concentration. When using toluene, an increase in the grafting percentage was observed until it stabilized after a 30% v/v concentration in the conditions used. This behavior occurs due to the gel effect, which consists of a self-acceleration of polymerization that is produced by an increase in the viscosity of the medium that makes termination reactions difficult, resulting in the formation of homopolymer gels rather than grafting.[22] Figure 3(c) shows the irradiation dose effect on the grafting percentage when using different concentrations (20, 30, and 40% v/v); in all cases, the graft percentage increased with increasing dose; it is also observed that at high doses of 40 and 50 kGy, there are no significant differences in the grafting percentage for 30 and 40% monomer concentrations.
Characterization
The material characterization was carried out by different assays. Initially, infrared spectroscopy was used to determine the presence of the functional groups of the matrix and the grafting monomer. Figure 2(a) shows the spectrum of pristine silicone catheters, which presents a band at 2964 cm−1 corresponding to the C-H stretching of the methyl groups, bands at 1257 cm−1 of the Si–C stretching, as well as two pronounced bands at 997 and 820 cm−1 of Si–O–Si stretching and Si–(CH3)2 bending.[23] The spectrum of PVNCL is also shown, which presents characteristic bands at 2923 cm−1 of C–H stretching, as well as bands own of the amide at 1625 cm−1 and of C–N stretching at 1479 cm−1.[24] The silicone catheters modified with PNVCL presented spectra that present the characteristic bands of both silicone and PNVCL. The amide and C–N stretching bands increased their intensity with increasing the PNVCL grafting percentage, which confirmed the grafting formation.
Figure 2(b) shows the thermal degradation of the pristine catheter, the PNVCL homopolymer, and the grafted catheters measured by TGA. The thermograms showed that the pristine silicone presents the highest thermal stability with a 10% weight loss at 507°C and a decomposition temperature at 662°C. In contrast, the PNVCL homopolymer showed the lowest thermal resistance with a loss of 10% by weight at 291°C and a decomposition temperature of 447°C. For their part, the grafting materials SRcat-g-PNVCL(25%) and SRcat-g- PNVCL(35%) presented a weight loss of 10% at temperatures of 442 and 422°C, and two decomposition temperatures at 456 and 626°C for those modified with 25% PNVCL and at 448 and 636°C for the modified ones with 35%. These results show the presence of the two polymers in the grafting materials.
On the other hand, Fig. 2(c) shows the results of the water swelling tests for the grafting materials; the test was carried out at 25°C. Silicone catheter is a hydrophobic material that does not interact with water, so it does not swell. However, the catheters grafted with PNVCL gained hydrophilicity, which increased with increasing grafting percentage. This behavior was also observed in the contact angle assays where the unmodified silicone catheters had angles of 103 ± 2°, while the grafted ones showed angles of 92 ± 2°, 100 ± 1°, and 93 ± 2° depending on the grafting percentage 3, 11, and 18%.
Finally, Fig. 2(d) shows the swelling in water of the grafted materials when varying the temperature. PNVCL is a thermo-responsive polymer whose homopolymer has an LCST between 32 and 34°C; however, this temperature can be displaced when PNVCL is copolymerized. The grafted silicone catheters with PNVCL, unlike the original catheter, showed thermo-response with an LCST at 29.5°C. This critical temperature is lower than the body temperature, so the system has the potential for the drug release in response to the stimulus.
Load and release of vancomycin
Vancomycin is an antibiotic used to treat infections caused by gram-positive bacteria, such as S. aureus. Vancomycin has functional groups in its structure that allow its interaction with the grafted material. The charge of vancomycin was determined by UV–Vis spectroscopy, it was observed that the materials obtained a maximum charge after 2 h of interaction with the solution of drug managing to load 1.1 ± 0.18 mg/g for SRcat-g-PNVCL (3%), 0.82 ± 0.02 mg/g for SRcat-g-PNVCL (10%), and 0.62 ± 0.09 mg/g for SRcat-g-PNVCL(18%). Figure 3 shows the release profiles of vancomycin from the grafting catheters. The three profiles were fitted to the Peppas-Sahlin model using the software DDsolver of Excel,[25] describing a release system controlled by two ways: drug diffusion and the relationship of the polymer chains.[26,27] The Eq. 5 shows the mathematical equation of the Peppas-Sahlin model:
where Mt/M is the rate between the amount of drug in determined time and the amount of drug in the equilibrium state, k1 is de Fickian diffusion contribution, k2 is the polymer chains relaxation contribution, and n is the Fickian diffusion exponent. The release constants of the materials shown in Table 1 indicate that the release is regulated by diffusion.
Although all materials carry a similar amount of vancomycin, the material grafted with 3% PNVCL is the one that allows the highest release, 93%, which was attributed to a lower interaction with the drug that allows the drug release. The amount of drug released decreased with increasing graft percentage, indicating an affinity increase between the drug and the grafted polymer, avoiding the diffusion of the drug.
Conclusions
PNVCL grafting was achieved on silicone catheters using the direct irradiation method, obtaining grafts in the range from 3 to 35%, depending on the conditions. Toluene turned out to be the best solvent for the grafting process, and maximum grafting was achieved with 30% monomer and 50 kGy. All the modified materials presented thermo-sensitivity and the ability to load and release vancomycin; however, the material with 3% PNVCL graft was the one that showed the highest release with a release percentage of 93%. These results show the antimicrobial potential of the grafted material.
Data availability
No applicable.
References
S.S. Magill, J.R. Edwards, W. Bamberg et al., Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 370, 1198–1208 (2014). https://doi.org/10.1056/NEJMoa1306801
P. Zarb, B. Coignard, J. Griskeviciene et al., The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 17, 1–16 (2012). https://doi.org/10.2807/ese.17.46.20316-en
L.E. Nicolle, Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 3, 1–8 (2014). https://doi.org/10.1186/2047-2994-3-23
M.A. Oyortey, S.A. Essoun, M.A. Ali et al., Safe duration of silicon catheter replacement in urological patients. Ghana Med. J. 57, 66–74 (2023). https://doi.org/10.4314/gmj.v57i1.10
M. Zare, E.R. Ghomi, P.D. Venkatraman, S. Ramakrishna, Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 138, 50969 (2021). https://doi.org/10.1002/app.50969
J.R. Henstock, L.T. Canham, S.I. Anderson, Silicon: The evolution of its use in biomaterials. Acta Biomater. 11, 17–26 (2015). https://doi.org/10.1016/j.actbio.2014.09.025
E. González, M.W. Frey, Synthesis, characterization and electrospinning of poly(vinyl caprolactam-co-hydroxymethyl acrylamide) to create stimuli-responsive nanofibers. Polymer (Guildf) 108, 154–162 (2017). https://doi.org/10.1016/j.polymer.2016.11.053
V. Kozlovskaya, E. Kharlampieva, Self-assemblies of thermoresponsive poly(N-vinylcaprolactam) polymers for applications in biomedical field. ACS Appl. Polym. Mater. 2, 26–39 (2020). https://doi.org/10.1021/acsapm.9b00863
L. Marsili, M. Dal Bo, G. Eisele et al., Characterization of thermoresponsive poly-N-vinylcaprolactam polymers for biological applications. Polymers (Basel) 13, 1–15 (2021). https://doi.org/10.3390/polym13162639
R.L. Sala, M.Y. Kwon, M. Kim et al., Thermosensitive poly(N-vinylcaprolactam) injectable hydrogels for cartilage tissue engineering. Tissue Eng. Part A 23, 935–945 (2017). https://doi.org/10.1089/ten.tea.2016.0464
J. Liu, A. Debuigne, C. Detrembleur, C. Jérôme, Poly(N-vinylcaprolactam): A thermoresponsive macromolecule with promising future in biomedical field. Adv. Healthc. Mater. 3, 1941–1968 (2014). https://doi.org/10.1002/adhm.201400371
E. Zavala-Lagunes, J.C. Ruiz, G.H.C. Varca, E. Bucio, Synthesis and characterization of stimuli-responsive polypropylene containing N-vinylcaprolactam and N-vinylimidazole obtained by ionizing radiation. Mater. Sci. Eng. C 67, 353–361 (2016). https://doi.org/10.1016/j.msec.2016.05.044
P. Singh, A. Srivastava, R. Kumar, Synthesis and characterization of nano micelles of poly(N-acrylamidohexanoic acid)-b-poly(N-vinylcaprolactam) via RAFT process: Solubilizing and releasing of hydrophobic molecules. Polymer (Guildf) 57, 51–61 (2015). https://doi.org/10.1016/j.polymer.2014.12.011
V.H. Pino-Ramos, A. Ramos-Ballesteros, F. López-Saucedo et al., Radiation grafting for the functionalization and development of smart polymeric materials. Top. Curr. Chem. 374, 1–28 (2016). https://doi.org/10.1007/s41061-016-0063-x
A.T. Naikwadi, B.K. Sharma, K.D. Bhatt, P.A. Mahanwar, Gamma radiation processed polymeric materials for high performance applications: A review. Front. Chem. 10, 1–15 (2022). https://doi.org/10.3389/fchem.2022.837111
A.M. Abdel-Ghaffar, Radiation synthesis and modification of biopolymers and polymeric composites for biomedical applications. Polym. Polym. Compos. 31, 1–13 (2023). https://doi.org/10.1177/09673911231166636
J.F. Monteiro, S.R. Hahn, J. Gonçalves, P. Fresco, Vancomycin therapeutic drug monitoring and population pharmacokinetic models in special patient subpopulations. Pharmacol. Res. Perspect. 6, 1–14 (2018). https://doi.org/10.1002/prp2.420
M.P. Wilhelm, Vancomycin. Mayo Clin. Proc. 66, 1165–1170 (1991). https://doi.org/10.1016/s0025-6196(12)65799-1
A. Maiti, W. Small, M.P. Kroonblawd et al., Constitutive model of radiation aging effects in filled silicone elastomers under strain. J. Phys. Chem. B 125, 10047–10057 (2021). https://doi.org/10.1021/acs.jpcb.1c04958
Q. Liu, W. Huang, B. Liu et al., Gamma radiation chemistry of polydimethylsiloxane foam in radiation-thermal environments: Experiments and simulations. ACS Appl. Mater. Interfaces 13, 41287–41302 (2021). https://doi.org/10.1021/acsami.1c10765
S. Hoca, N. Olacak, Y. Anacak, The interaction of ionizing radiation and silicone medical implants: Evaluation of the effects on radiotherapy dose distribution and alteration in the physical properties of the silicone. Radiat. Phys. Chem. 208, 1–9 (2023). https://doi.org/10.1016/j.radphyschem.2023.110893
J.W. Gooch, Autoacceleration, in Encyclopedic Dictionary of Polymers. ed. by J.W. Gooch (Springer, New York, 2011), pp.55–56
J.M. Cornejo-Bravo, K. Palomino, G. Palomino-Vizcaino et al., Poly(N-vinylcaprolactam) and salicylic acid polymeric prodrug grafted onto medical silicone to obtain a novel thermo- and pH-responsive drug delivery system for potential medical devices. Materials (Basel) 14, 1–17 (2021). https://doi.org/10.3390/ma14051065
T.Ö. Selin Kozanoǧlu, A. Usanmaz, Polymerization of N-vinylcaprolactam and characterization of poly(N-vinylcaprolactam). J. Macromol. Sci. Part A 48, 467–477 (2011). https://doi.org/10.1080/10601325.2011.573350
Y. Zhang, M. Huo, J. Zhou et al., DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 12, 263–271 (2010). https://doi.org/10.1208/s12248-010-9185-1
W.B. Liechty, D.R. Kryscio, B.V. Slaughter, N.A. Peppas, Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 1, 149–173 (2010). https://doi.org/10.1146/annurev-chembioeng-073009-100847
M.L. Bruschi, 5-Mathematical models of drug release. In: Strategies to Modify the Drug Release from Pharmaceutical Systems. Woodhead Publishing, Sawston, pp. 63–86 (2015)
Acknowledgments
The authors thank B. Leal and M. Cruz from ICN-UNAM for their technical assistance.
Funding
This work was supported by Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México under Grant IN204223.
Author information
Authors and Affiliations
Contributions
Conceptualization: MUR-F, LD-P, and EB; Methodology: MUR-F and LD-P; Formal analysis and investigation: LD-P and EB; Writing—original draft preparation: LD-P and EB; Writing—review and editing: LD-P and EB; Funding acquisition: EB; Resources: EB; and Supervision: EB.
Corresponding authors
Ethics declarations
Competing interest
The authors have no competing interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramirez-Fuentes, M.U., Duarte-Peña, L. & Bucio, E. Drug delivery in thermo-responsive silicone catheters by grafting of N-vinylcaprolactam using gamma radiation. MRS Communications 14, 311–316 (2024). https://doi.org/10.1557/s43579-024-00528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-024-00528-5