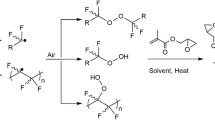
Abstract
The aim of this study was to develop a drug delivery copolymer. Hydroxyethyl methacrylate (HEMA) was grafted onto poly(tetrafluoroethylene) films using gamma rays as the initiator agent. The optimal conditions for the grafting procedure were determined through a series of experiments, considering the following factors: reaction time, temperature, HEMA concentration, solvent type, and gamma-ray doses. Grafting was accomplished using the oxidative-pre-irradiation method. The resulting copolymer was subjected to various characterizations, including thermal analysis (TGA and DSC), FTIR-ATR spectroscopy, contact angle measurements, and assessment of physicochemical properties. Additionally, its ability to load and release ciprofloxacin was evaluated.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In recent years, studies on materials modification have witnessed a remarkable rise within the scientific community; this is due to their significant implications as a problem-solving alternative across various domains. In many fields, the utilization of polymers has surpassed that of other materials[1] owing to their exceptional processability and versatility.[2] Among these polymers, there are some that exhibit stimuli-sensitive responses, endowing them with the capability to serve as drug delivery systems. This property allows them to release a load when exposed to specifics triggers. This mechanism offers several advantages, including limiting drug diffusion into healthy tissues and preventing the generation of bacterial resistance.[3,4] Consequently, this approach enhances drug bioavailability[5] while reducing the required dosage.[6]
Despite having those remarkable properties, not all polymers have the desirable qualities for a specific goal and even more they could have unfavorable characteristics. However, it is possible to modify its internal structure and properties, by adding grafting monomer to its structure; this could be done using free radicals and interpenetrate chains, which would allow the attachment of external structures into another.[7,8]
Among different ways of inducing free-radical formation, the use of gamma rays has attractive characteristics, such as a high ionization power, which allows a deep intrusion into the sample, reduces the loss of mechanical properties, do not produce many collateral residues, sterilizes the sample, and leads to an uniform radical formation and homogeneous grafting over the surface.[9,10,11]
For the main polymer, PTFE was selected by reasons of its good mechanical properties, great thermal and chemical stability, low biological reactivity, anti-adherent surface, high flexibility, resistance against deformation, and low friction coefficient.[12,13] Nevertheless, its hydrophobic character places it in a bad situation against bacterial degradation.[14] To cover this deficit, HEMA was chosen as the adding structure; because among other things, it shows a hydrophilic character, antimicrobial properties, and hydrogels generation and has previously been successfully added to other structures.[15,16,17,18] It was well known that when working with HEMA, its high reactivity had to be considered;[19] however, some studies showed that when diluted in a solvent with certain amount of methanol, it is possible to work with high concentrations of HEMA within short reaction time.[20,21] Methanol was also considered, because it leads to a predominately superficial grafting.[22,23,24] Finally, it was considered not to exceed 20 kGy and 65°C to prevent any PTFE damage.[25]
The aim of this study was to graft HEMA onto PTFE to obtain a copolymer with mixed properties, and HEMA was chosen to attempt to modify PTFE hydrophilicity and to try to create a superficial yield over PTFE. The grafting was carried out following the pre-irradiation method and the resulting structure PTFE-g-HEMA was tested for its swelling degree, transitions and decomposition temperatures, hydrophobicity, internal composition, and lastly for its capability to load and release ciprofloxacin, a fluoroquinolone antibiotic.
Materials and methods
Materials
PTFE films with a thickness of 100 µm were purchased from Goodfellow Sigma-Aldrich. Hydroxyethyl methacrylate (HEMA) was purchased from Sigma-Aldrich and purified under vacuum distillation. All solvents such as methanol, ethanol, and toluene were obtained from J.T. Baker, and ciprofloxacin from Sigma-Aldrich (St Louis, MO, USA).
Synthesis of PTFE-g-HEMA using the oxidative pre-irradiation method
Films of PTFE (1 × 5 cm) were placed inside a borosilicate test tube and exposed to gamma rays from a 60Co source in presence of oxygen from the air. Using a dose rate of 17.1 kGy/h, films were irradiated from 5 to 20 kGy. Once the samples were pre-irradiated, 8 ml of solution with different HEMA concentrations (5–30 vol.%) were added. These solutions were prepared using whether water, methanol, ethanol, and toluene depending on the treatment. The graft polymerization systems were subjected to argon bubbling for 15 min to displace the air, then sealed, and heated up to temperatures ranging from 30 to 70°C over predetermined reaction times (2–6 h). Finally, the newly obtained materials were washed with methanol for 24 h to remove any homopolymer formation and dried up under vacuum at 40°C until a constant weight was reached. The graft percentage was calculated using the following equation:
where \(Wo\) and \(Wg\) correspond to the weights of the samples before and after the grafting, correspondingly.
Characterization
The study of the inner structure of the new copolymer was performed by FTIR-ATR, reading between 4000 and 650 cm−1. Its thermal characterization was got by TGA and DSC analyses; a heating rate of 10°C/min was used in both cases. TGA was used to elucidate its thermal degradation temperatures in a range of 25 to 800°C, whereas DSC showed its thermal transitions in a range of 25 to 400°C.
Contact angle
The contact angle of PTFE-g-HEMA samples was measured at room temperature using a goniometer (DSA 100, Krüss GmbH, Germany). The flat and dry films were placed inside the chamber and a drop of distilled water was deposited on the surface of each sample. The contact angle was recorded using the computer software at various time intervals (0–5 min). Each experiment was conducted in triplicate.
Swelling degree
PTFE-g-HEMA films, previously weighed, were immersed in distilled water at 25°C. The samples were removed from the water, excess of water was removed using paper towels, measured at predetermined time intervals, and then samples were re-immersed in distilled water. The degree of swelling is calculated using the following equation:
where \(Ws\) and \(Wd\) are the weights of the swollen and dried films, respectively.
Drug loading and release
Ciprofloxacin loading was conducted using previously washed PTFE-g-HEMA (11.8%) films, which were dried under vacuum conditions until a constant weight was reached. The samples were immersed in vials containing 3 ml of an aqueous ciprofloxacin solution (12 μg/ml) under mechanical stirring; the loading process was recorded at room temperature under fixed time intervals. Samples were removed from the solution and through an UV absorbance measurement at 270 nm, the concentration of loaded drug from the solution was calculated using a calibration curve, whose equation is showed below.
where \(x\) is the absorbance at 270 nm at a certain time.
Therefore to calculate the drug loaded per area, the following equation was used. The experiment was performed in triplicate.
where \({Dc}_{i}\) and \({Dc}_{f}\) are the initial and final concentration of the loading medium in µg/ml at an established time, \(V\) is the volume of the medium in ml, and A is the area of the sample in cm2.
Ciprofloxacin release was monitored by placing the loaded films of PTFE-g-HEMA (11.8%) into amber vials containing 3 ml of phosphate buffer pH 7.4 at 37°C with moderate mechanical stirring. The released ciprofloxacin was measured at specific time intervals by removing a sample from the release medium and measuring the UV absorbance of the solution at 270 nm. The concentration of released drug from the films at fixed intervals was calculated using a calibration curve, whose equation is showed below.
where \(x\) is the absorbance at 270 nm at a certain time.
Afterward to calculate the drug released per area, the following equation is used. The experiment was conducted in triplicate.
where \(Dr\) is the concentration of drug releasing medium in µg/ml at established time and \(V\) is the volume in ml where the drug was released and \(A\) is the area of the sample in cm2.
Instrumental
Vacuum drying
Oven model Yamato ADP21 was employed to store the samples before its use (at least for 24 h) under conditions of 25°C and 0.1 kPa.
Contact angle
Kruss DSA 100 drop shape analyzer (Matthews, North Carolina, USA) was used to measure the contact angle.
Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR)
Dry samples were analyzed using a Perkin–Elmer Spectrum 100 spectrometer (Norwalk, Connecticut, USA) of 16 scans. TA Universal analysis software was used for data.
Thermogravimetric analysis (TGA)
Weight loss and decomposition of films were observed by heating at a rate of 10°C min−1 and running from 25 to 800°C in a TGA instrument Q50 TA Instruments (New Castle, Delaware, USA).
Differential scanning calorimetry (DSC)
Differential scanning calorimetry of films was performed by heating at a rate of 10°C min−1 and running from 25 to 400°C in a DSC instrument Q50 TA Instruments (New Castle, Delaware, USA).
Ultraviolet–visible (UV–vis)
Spectrophotometer model SPECORD 200 plus from Analytic, Germany, which used quartz cuvettes of 1 cm in width.
Results
Grafting polymerization of HEMA on PTFE films
The grafting of HEMA was carried out by pre-irradiation oxidative method (Fig. 1) to generate peroxides and hydroperoxides on the PTFE matrix; those are reactive species that act as radical formation initiators when exposed to high temperatures. The grafting polymerization was initially evaluated employing different solvents. The results revealed the significant influence of the type of solvent over the grafting degree (Fig. 2). A complex behavior was observed, where the highest grafting degrees were achieved when using toluene and water, they reached approximately a graft of 5%, while ethanol and methanol led to grafting degrees of less than 1%. This suggests that alcohols promote homopolymerization of HEMA. However, it was seen that the usage of pure water increased the damages over PTFE films. Building on these findings, methanol and water were chosen as the more optimal solvents for subsequent studies. The methanol–water ratio was varied while maintaining a constant monomer concentration of 20 vol.%. The outcome indicated a decrease in grafting degree when increasing methanol percentage.
Grafting yields as function of different applied doses. Synthesis conditions were as follows: (a) irradiation: 12 kGy, temperature: 60°C, reaction time: 2 h, HEMA at 20 vol.%, and solvent: variable. (b) Irradiation: 12.5 kGy, temperature: 50°C, reaction time: 3 h, HEMA 20 vol.%, and solvent: methanol/water at different concentrations. (c) Irradiation: 10 kGy, temperature: 60°C, reaction time: 2 h, solvent: water, and HEMA at different concentrations. (d) Temperature: 60°C, reaction time: 2 h, HEMA 20 vol.%, solvent: water, and irradiation: variable. (e) Irradiation: 10 kGy, reaction time: 3 h, HEMA 30 vol.%, solvent: water, and temperature: variable. (f) Irradiation: 12.5 kGy, temperature 55°C, HEMA 25 vol.%, solvent: methanol/water 15 vol.%, and reaction time: variable.
The grafting polymerization was conducted with the variation of other parameters, such as temperature, reaction time, monomer concentration, and absorbed dose. These variables collectively exhibited a direct correlation with the grafting percentage, showing a steady increase in graft percentage (Fig. 2). The exploration of grafting polymerization under these varied conditions aimed to optimize the grafting degree while preserving the original properties of the PTFE matrix as much as possible. The results indicated that higher temperatures lead to a reduction in reaction time but an increase in damage. At 70°C, the sample completely lost its original matrix properties. Consequently, temperature ranges between 55 and 60°C were chosen, with reaction times of 2–3 h. As a result, PTFE-g-HEMA films with grafting percentage of 11.8% were the highest graft percentage that quite fully kept the properties of the matrix, such as its resistance. The damage over PTFE films is not fully correlated to one specific variable but the sum of them; mechanical loss properties had low correlation with the amount of graft percentage; some grafts could be little higher than others but with less degradation or modification of the film. A predominantly superficial graft was favored by the use of water which does not embed into PTFE [Fig. 1(a–d)]. Copolymers over 16 graft percentage suffered severe damages; some of them were even tore as is seen in the previous figure [Fig. 1(e)].
FTIR-ATR analysis
FTIR-ATR analysis confirmed the surface modification of the matrix after carrying out the grafting polymerization of HEMA, promoted by gamma rays. The spectra of PTFE films [Fig. 3(a)] showed the bands belonging to the vibrations of C–F bonds of PTFE at 1200–1145 cm−1, while the spectra of PTFE-g-HEMA films show the corresponding bands to PHEMA grafted onto PTFE film. PHEMA significant peaks were assigned to –OH (3420 cm−1), C–H (2946 cm−1), C=O (1720 cm−1), and C–O (1450 cm−1).[26] These results indicated the successful synthesis of the graft copolymer.
(a) FTIR-ATR spectra of PTFE with different grafting percentages and PHEMA. (b) TGA and (c) DSC analyses under nitrogen atmosphere. (d) Swelling kinetic of PTFE films with different grafting percentages in distilled water at 25°C. (e) Contact angle of PTFE films modified with PHEMA grafting at 25°C in 1 min.
Thermal characterization
TGA and DSC thermal analyses were conducted over samples with different grafting percentages.
The results from TGA [Fig. 3(b)] indicated a straightforward thermal degradation pattern for PTFE films at 567.97°C. On the other hand, the films grafted with PHEMA displayed a dual-degradation patrons, the first slope at 290.50°C corresponds to the PHEMA grafted onto PTFE films, followed by the thermal degradation of the matrix at 583.80°C. The initial thermal decomposition of PTFE-g-HEMA is well correlated with the gravimetrically determined grafting percentages; its decomposition temperature exceeded that of PHEMA (243.88°C). These findings demonstrated that the grafted polymers increased its thermal stability, due to the inherent thermal stability conferred by the PTFE matrix. In summary, all samples exhibited high degradation temperatures. HEMA-grafted samples displayed a 10% weight loss temperature of 532.70°C for PTFE-g-HEMA (4.8%) and 332.8°C for PTFE-g-HEMA (16.1%).
Results from the DSC analysis revealed the decomposition temperature (Td) of PTFE at 330.28°C, while PHEMA exhibited a glass transition temperature around 109.7°C[27] and a second peak-like feature at 231.74°C, attributed to thermal degradation. Notably, DSC analysis of PTFE-g-HEMA films with graft percentages of 4.8 and 16.1 displayed two transitions, the first one of 210.04°C for a graft of 4.8% and 228.78°C for 16.1%, possibly indicating a chemical reaction preceding thermal degradation, followed by the second peak at 326.01 and 325.88°C for each, respectively, graft percentages, belonging to the melting point of PTFE [Fig. 3(c)].
Swelling and contact angle studies
The swelling study was conducted in distilled water at room temperature using PTFE-g-HEMA films with variable graft percentages. The results revealed a clear correlation between graft percentage and swelling behavior, the higher the graft percentage, the higher the swelling percentage. Maximum swelling was observed after 10 min, highlighting the strong affinity of PHEMA, over the matrix, for hydrophilic solvents. This affinity altered the surface properties of the PTFE matrix, causing a substantial increase in swelling from 0.3 to 3.6% [Fig. 3(d)].
Furthermore, the contact angle study [Fig. 3(e)] revealed a slight increase in hydrophilicity of the surface of PTFE. With a graft percentage of 16.1%, the contact angle shifted from 104.8° (hydrophobic) to 86° (hydrophilic by definition), while PTFE-g-HEMA with a 4.8% graft percentage exhibited a contact angle of 91.5°. Additionally, these contact angles remained relatively stable over a 5-min interval. The results clearly indicated the ability to regulate surface hydrophilicity through graft percentage manipulation, the increase of PHEMA grafting, leads to a modification of the contact angle.
Drug loading and release
The ciprofloxacin loading studies were conducted in distilled water, using PTFE-g-HEMA films with grafting percentages of 0 and 11.8. The ciprofloxacin loading of PTFE-g-HEMA (11.8%) films exhibited a rapid saturation, within 60 min, followed by a slow continuous loading of the drug; its maximum load was 1.79 µg/cm2 within 240 min. A similar behavior was observed for PTFE films, which reached its maximum load at 1.02 µg/cm2 at the same time [Fig. 4(a)]. These results clearly demonstrate the effect of PHEMA grafting on ciprofloxacin loading, leading to an increase in drug uptake due to the enhanced surface affinity of the new material, likely attributed to new chemical interactions involving hydrogen bonding between the graft polymer and the drug.
The ciprofloxacin release studies were conducted in a phosphate-buffered saline (pH 7.4) at 37°C. The results showed that the PTFE films reached a fast state of release equilibrium within the initial minutes; its maximum release was 0.33 µg/cm2 over 240 min, which represents 32.43% of the maximum load. In the case of the PTFE-g-HEMA film (11.8%), it exhibited a similar behavior with a first peak at 60 min with a release of 0.51 µg/cm2 of ciprofloxacin, followed by a steady release until its maximum peak at 0.59 µg/cm2 within 240 min, representing 33.42% of its maximum load. Therefore, the grafting of PHEMA onto PTFE films allowed higher release of ciprofloxacin compared to the PTFE films, which rapidly reached the plateau without further exponential increase in release. Nevertheless, accordingly to the CIPRO medication guide from Bayer HealthCare Pharmaceutical Inc., the obtained ciprofloxacin release is below the minimum inhibitory concentration in a human body, but when used locally, this concentration could inhibit the proliferation of potentially harmful microorganisms. Its fast drug release could be useful for wound healing treatments, based on the PTFE-g-HEMA film (11.8%) release of 66% within 15 min.
Conclusion
The graft polymerization of HEMA onto a PTFE matrix was successfully achieved through the oxidative pre-irradiation method. This generated appropriate chemical groups that acted as initiators for the graft polymerization of HEMA under the suitable conditions of monomer concentration, solvent, and reaction temperature. These factors do not only enabled the graft polymerization on the PTFE films but also effectively controlled the degree of grafting. The newly obtained materials also exhibited an enhancement in the loading and release of ciprofloxacin, providing sustained behavior. This demonstrates a potential application as an antimicrobial wound dressing material, mitigating potential medical complications arising from the presence of pathogenic microorganisms.
Data availability
Not applicable.
References
D.K. Mandal, H. Bhunia, P.K. Bajpai et al., Optimization of acrylic acid grafting onto polypropylene using response surface methodology and its biodegradability. Radiat. Phys. Chem. 132, 71–81 (2017). https://doi.org/10.1016/J.RADPHYSCHEM.2016.12.003
S. Yang, K.-F. Leong, D. Zhaohui, The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 7(6), 679–689 (2001). https://doi.org/10.1089/107632701753337645
W.C. Reygaert, An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4(3), 482–501 (2018). https://doi.org/10.3934/microbiol.2018.3.482
J. Khandare, T. Minko, Polymer–drug conjugates: progress in polymeric prodrugs. Prog. Polym. Sci. 31(4), 359–397 (2006). https://doi.org/10.1016/J.PROGPOLYMSCI.2005.09.004
G.B. Heggannavar, D. Achari, C. Fernandes, G. Mitchell, P. Morouço, M.Y. Kariduraganavar, Smart polymers in drug delivery applications. Publ. online (2019). https://doi.org/10.4028/www.scientific.net/AMM.890.324
N. Seedat, R.S. Kalhapure, C. Mocktar et al., Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: in vitro and in silico studies. Mater. Sci. Eng. C 61, 616–630 (2016). https://doi.org/10.1016/J.MSEC.2015.12.053
E. Bucio, G. Burillo, Radiation-induced grafting of sensitive polymers. J. Radioanal. Nucl. Chem. 280(2), 239–243 (2009). https://doi.org/10.1007/s10967-009-0505-9
A.F. Saad, M.H. Ibraheim, A.M. Nwara, S.A. Kandil, Modifications in the optical and thermal properties of a CR-39 polymeric detector induced by high doses of γ-radiation. Radiat. Phys. Chem. 145, 122–129 (2018). https://doi.org/10.1016/J.RADPHYSCHEM.2017.10.011
Y.S. Ramírez-Fuentes, E. Bucio, G. Burillo, Radiation-induced grafting of N-isopropylacrylamide and acrylic acid onto polypropylene films by two step method. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 265(1), 183–186 (2007). https://doi.org/10.1016/j.nimb.2007.08.046
Y. Kodama, M. Barsbay, O. Güven, Poly(2-hydroxyethyl methacrylate) (PHEMA) grafted polyethylene/polypropylene (PE/PP) nonwoven fabric by γ-initiation: synthesis, characterization and benefits of RAFT mediation. Radiat. Phys. Chem. 105, 31–38 (2014). https://doi.org/10.1016/J.RADPHYSCHEM.2014.05.023
A.R. Hernández-Martínez, E. Bucio, Novel pH- and temperature-sensitive behavior of binary graft DMAEMA/PEGMEMA onto LDPE membranes. Des. Monomers Polym. 12(6), 543–552 (2009). https://doi.org/10.1163/138577209X12478293300757
J.G. Drobny, Technology of fluoropolymers, 2nd edn. (Taylor and Francis Group, Berlin, 2009)
J. Lochab, V.R. Singh, Acoustic behaviour of plastics for medical applications. Indian J. Pure Appl. Phys. 42, 595–599 (2004)
J. Park, R.S. Lakes, Biomaterials: an introduction, 3rd edn. (Springer Science + Business Media LIC, Berlin, 2007)
S. Rattan, T. Sehgal, Stimuli-responsive membranes through peroxidation radiation-induced grafting of 2-hydroxyethyl methacrylate (2-HEMA) onto isotactic polypropylene film (IPP). J. Radioanal. Nucl. Chem. 293(1), 107–118 (2012). https://doi.org/10.1007/s10967-012-1728-8
S.L. Tomić, M.M. Mićić, S.N. Dobić, J.M. Filipović, E.H. Suljovrujić, Smart poly(2-hydroxyethyl methacrylate/itaconic acid) hydrogels for biomedical application. Radiat. Phys. Chem. 79(5), 643–649 (2010). https://doi.org/10.1016/J.RADPHYSCHEM.2009.11.015
K. Sudhakar, K. Madhusudana Rao, M.C.S. Subha, K. Chowdoji Rao, E.R. Sadiku, Temperature-responsive poly(N -vinylcaprolactam-co-hydroxyethyl methacrylate) nanogels for controlled release studies of curcumin. Des. Monomers Polym. 18(8), 705–713 (2015). https://doi.org/10.1080/15685551.2015.1070497
Y. Guadarrama-Zempoalteca, L. Díaz-Gómez, H.I. Meléndez-Ortiz, A. Concheiro, C. Alvarez-Lorenzo, E. Bucio, Lysozyme immobilization onto PVC catheters grafted with NVCL and HEMA for reduction of bacterial adhesion. Radiat. Phys. Chem. 126, 1–8 (2016). https://doi.org/10.1016/j.radphyschem.2016.04.023
A. Khan, T. Huq, R.A. Khan, D. Dussault, S. Salmieri, M. Lacroix, Effect of gamma radiation on the mechanical and barrier properties of HEMA grafted chitosan-based films. Radiat. Phys. Chem. 81(8), 941–944 (2012). https://doi.org/10.1016/J.RADPHYSCHEM.2011.11.056
L.M. Ferreira, J.P. Leal, P.A. Rodrigues, L.C. Alves, A.N. Falcão, M.H. Gil, Characterization of PE-g-HEMA films prepared by gamma irradiation through nuclear microprobe techniques. Radiat. Phys. Chem. 81(9), 1319–1323 (2012). https://doi.org/10.1016/J.RADPHYSCHEM.2012.01.045
G. González-Hernández, V.H. Pino-Ramos, L. Islas, C. Alvarez-Lorenzo, A. Concheiro, E. Bucio, Radiation-grafting of N-vinylcaprolactam and 2-hydroxyethyl methacrylate onto polypropylene films to obtain a thermo-responsive drug delivery system. Radiat. Phys. Chem. 2019(157), 6–14 (2017). https://doi.org/10.1016/j.radphyschem.2018.12.014
S. Cabana, C.S. Lecona-Vargas, H.I. Meléndez-Ortiz et al., Silicone rubber films functionalized with poly(acrylic acid) nanobrushes for immobilization of gold nanoparticles and photothermal therapy. J. Drug Deliv. Sci. Technol. 42, 245–254 (2017). https://doi.org/10.1016/j.jddst.2017.04.006
W.A. Eckert, D. Groöbe, U. Rothe, Surface-modification of polystyrene-microtitre plates via grafting of glycidylmethacrylate and coating of poly-glycidylmethacrylate. Biomaterials 21(5), 441–447 (2000). https://doi.org/10.1016/S0142-9612(99)00098-8
Y.A.E. Lozano, Injerto de Metacrilato de Metilo y 2-(Dietilamino) Etil Metacrilato En Películas de Politetrafluoroetileno Mediante Radiación Ionizante (Universidad Nacional Autonoma de México, Mexico City, 2019)
S.A.V. Lozada, Síntesis de Copolímeros de Injerto En Películas de Politetrafluoroetileno Con Ácido Acrílico, Ácido Metacrílico y 4-Vinilpiridina (Universidd Nacional Autonoma de México, Mexico City, 2019)
N. Xu, M. Ren, H. Cheng, Sol-modified sintering-induced mesoporous polytetrafluoroethylene/poly (acrylic acid-co-hydroxyethyl methacrylate) composite fiber as an adsorbent with high adsorption capacity for dyes. Mater. Chem. Phys. 272, 124988 (2021). https://doi.org/10.1016/j.matchemphys.2021.124988
H. Sahabudeen, R. Machatschek, A. Lendlein, Thermal behavior of poly(2-hydroxyethyl methacrylate-bis-[trimethoxysilylpropyl]amine) networks. IOP Conf. Ser. (2013). https://doi.org/10.1088/1757-899X/45/1/012005
Acknowledgments
The authors thank Benjamin Leal, Martín Cruz, Lorena Duarte, and Gabriel Flores from ICN-UNAM, for their technical assistance.
Funding
This work was supported by the Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México under Grant IN204223.
Author information
Authors and Affiliations
Contributions
Conceptualization: BER-F and EB; Methodology: BER-F; Formal analysis and investigation: BER-F and EB; Writing and original draft preparation: BER-F, EB; Writing, reviewing, and editing of the manuscript: BER-F and EB; Funding acquisition: EB; Resources: BER-F and EB; Supervision: EB.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramírez-Flores, B.E., Bucio, E. Synthesis and characterization of the graft copolymer PTFE-g-HEMA using gamma rays for the load and delivery of ciprofloxacin. MRS Communications 14, 26–33 (2024). https://doi.org/10.1557/s43579-023-00491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-023-00491-7