Abstract
The escalating curiosity in bacterial cellulose (BC) due to exceptional attributes such as purity, biodegradability, non-toxicity, porous fibrillar structure, and high water retention potential expand its applications to tissue engineering, controlled drug delivery, and cosmetics. BC has proved highly prospective to be used to manufacture innovative wound care solutions, drug carriers and delivering complexes. The drug-carrying BC found enormous applications in dental therapies, wound care, and scare-free wound management. Various degradation techniques of BC under antibiotic environments and physiological conditions offer different advantages in drug design. The drug loading capacity of BC can be increased by in situ modifications of its fibrillar network. The BC-based scaffolds compounded with other materials such as nanopolymers have explored new frontiers for BC applications in auspicious biomedicinal product developments. BC can accommodate different nanoparticles, biomaterials, synthetic materials, carbon materials, and plant extracts, which allows using BC in various biomedical and cosmetic products.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotechnology is a group of techniques for offering several modern natural innovative materials, and bacterial cellulose (BC) is one of these imaginative bio-materials produced through a biotechnological process by different bacteria strains from glucose [1]. Various regulatory bodies are compelled to strictly follow the application of xeno-free biomaterials in human health care, especially in drug delivery; bacterial cellulose can cater to these requirements due to its animal-free and human-free origin. Bacterial cellulose can be produced by several bacteria, such as Salmonella, Escherichia, Agrobacterium, Achromobacter, Rhizobium, Aerobacter, Azotobacter, Sarcina, and Gluconacetobacter [2]. BC can grow in a supervised culture environment, either dynamic perfusion bioreactors or conventional static cell culture conditions. The cost of culture medium is one-third of the cost of BC production, driving it to explore alternative low-cost carbon and nitrogen sources of culture media. BC can adopt fascinating shapes like fleeces, fibrous aggregates, foils, spheres, tubes, and films. Apart from that, needle-shaped nanocrystalline structures can also be obtained by degradation of the amorphous region by oxidative or enzymatic degradation [3, 4]. Like plant cellulose, BC also shares the typical chemical formula, biodegradation features, and high chemical resistance. Nevertheless, BC’s distinctive three-dimensional fibrillar network provides superior properties to plant cellulose like very high Young’s modulus: 2GPa, thermal stability upto 300 °C, crystalline content 70–90%, moisture content higher than 90%, degree of polymerization upto 10,000, and outstanding formability. Besides some limitations in bio-persistency, elongation, and fineness considered for exhaustive nanosafety discussions, BC has secured enough acceptability to be a highly biocompatible material [5, 6].
These bacterial cellulose-based natural composites have a high potential to suppress microbial growth, promote wound healing, to provide controlled-release oral drugs. This database will help with better biocompatibility, zero cytotoxicity, and higher wellness in future product design. Bacterial cellulose's biosynthesis, properties and applications are different from plant cellulose. Bacterial cellulose is an aggregate of cellulose nanofibrils secreted extracellularly by some bacterial strains through the bottom-up synthesis process, while the plant nanocellulose is derived from the top-down mechanical and chemical procedure [7, 8]. Plant cellulose is isolated by separating from hemicelluloses and lignin through highly polluting chemical processes, although BC purification requires minimum energy [9]. The purification requires removing hemicelluloses, pectin, lignin, and colouring material in plant cellulose. However, BC purification involves the death of microorganisms and the removal of cell waste and culture medium from the cellulose matrix [10].
Fewer fibrils with a higher diameter with weak hydrogen bonding are part of plant cellulose, while more fibrils with a smaller diameter with stronger hydrogen bonding are present in BC [11]. The biosynthesis environment, parameters, medium and bacterial strain mainly engineers the structure of BC. This paper critically reviews research carried out over the last 5 years on bacterial cellulose and its application to explore antimicrobial, cosmetic, and controlled drug release potentials.
Engineering of BC Structure
BC has compact, flexible, and three-dimensional networks of fine fibrils. The existence of hydrogen bonding in BC influences the morphology significantly. The existence of hydrogen bonding influences several BC activities, like chemical modification, polymerization or depolymerization, and solubilization. Sometimes, these hydrogen bonds link with other molecules, such as ethylene glycol or glycerol, where extreme hydrogen bonding guides to engineering high sorption affinity, providing it with a hydrophilic entity [12]. The dissolution of BC has always remained challenging due to strong hydrogen bonding and high crystallinity. The N-methyl morpholine N-oxide (NMMO) is proven as an effective solvent of BC by reducing the crystallinity from 79 to 38% due to the massive saluting action of NMMO [13]. The BC has higher mechanical strength than plant cellulose due to its high crystallinity. The elastic modulus of single crystal BC was 78 GPa by atomic force microscopy and 114 GPa by Raman spectroscopy. The BC has a low elastic modulus of 15–35 GPa in sheet form. The bursting strength of BC films enhances at an elevated oxygen ration by increasing the BC layers and thickness of the individual layer in a tube-like structure. The biosynthesis of bacterial cellulose, consisting of pertinent fibrils, surrounds a nucleating point and gets gathered to form the pellicle, which needs many genes whose products carry out the synthesis of nanofibrillar cellulose and its secretion [14,15,16]. However, the essential genes required for synthesizing bacterial cellulose were not established in the past. Recently, it confirmed that the production of bacterial cellulose pellicle is confirmed by transferring an essential set of genes into other bacteria [17]. These operator genes within the BCs (bacterial cellulose synthesis) consist typically of bcsA, bcsB, bcsC, bcsD, cmcax and ccpAx. The bit part of different genes is observed, where bcsA produces the catalytic subunits of bacterial cellulose, bcsB creates the regulatory subunit of the enzymes, which binds with cyclic di-guanylic acid (cyclic di-GMP: second messenger utilized in signal transduction in a broad diversity of bacteria) [18]. The cellulose synthase task of the bcsA subunit can accordingly be allosterically controlled by cyclic di-GMP control of the bcsB switch. The gene bcsC forms the membrane channels used for the secretion of cellulose, while bcsD is known to produce cellulose in crystalline fibrillar form. The downstream gene cmcax that cryptography for endo-beta-1,4glucanase, which is excreted into extracellular volume and is credited to effect the gathering of cellulose ribbons in case of failure in positioning by splitting snarled cellulosic chains [19].
The gene ccpAx product is responsible for locating the bcs complex to the cell membrane and interconnecting with the bcsD subunits. To yield uridinediphosphate (UDP)-glucose, the cell must have the usual enzyme of glucose kinase to produce glucose-6-phosphate from glucose, phosphoglucomutase to isomerize glucose-6-phosphate, glucose-6-phosphate to glucose-1-phosphate, and UDP-glucose pyrophosphorylase to form UDP-glucose from UTP and glucose-1-phosphate. The most straight forward bacterial cellulose synthesis pathway in Acetobacter xylinum is given in Figure 1. A variety of carbon sources as fructose, glucose, and galactose, may enter the BC biosynthesis pathway [20]. Different bacterial species have privileged carbon sources. The carbon sources significantly influence the synthesis rate and yield of BC. The BC production rate depends on the polymerization rate of UDP-glucose into β linked chains regulated by this enzyme. In the absence of cyclic diguanylate (cyclic-di-GMP) an allosteric activator of cellulose synthase leads to zero production of BC. Indeed, cyclic-di-GMP reversibly ties to a membrane protein: cyclic-di-GMP binding protein and becomes inaccessible, hence, managing the balance between bound and unbound cyclic-di-GMP. The intracellular potassium concentration is crucial in enhancing BC production [21]. The transformation of only bcsABC genes results in an amorphous BC with zero application as a biomaterial. The existence of van der Waals forces initially assists in crystallizing cellulose molecular chain into mini-sheet or arrays, and hydrogen bonds of the mini-sheets into the mini crystal which emerge from the bacterial membrane in the form of a single terminal complex subunit which is orderly arranged to get crystalline fibrils. BC biosynthesis pathway with metabolite is given in Figure 1.
As pictorially shown in Figure 2, the microfibrils’ additional structuring into microfibrillar bundles by the adequately intimate closeness of adjacent TC subunits into an active row of TCs and eventually, ribbon formation becomes possible. The BC creation process follows the remarkable hierarchy in the cellulose assembly process, which is primly regulated by bcsD controlled arrangement of linear TC sheets and their alignment towards the cell axis [22]. The systematically organized 3D BC network offers good tensile strength, porosity, and stability and offers a wide range of applications, which restricts by its relatively slow manufacturing rate and high cost than plant cellulose.
The bacterial cellulose manufacturing process needs culture conditions, static or agitated bioreactor conditions, with growth media mainly the carbon source, other nutrients at appropriate pH, and appropriate bacterial strain. The oxygen transfer coefficient of culture directly affects the BC production yield. BC production is an aerobic process that happens primarily at the assemblage of air, the medium of static culture. The high production yield of BC is reported in the agitated process due to the availability of over-dissolved oxygen in the medium [23]. The culture medium's stirring degree enhances the homogeneous oxygen distribution with high availability but produces BC pellicles of lower mechanical strength than static conditions [24]. The BC synthesis from Acetobacter xylinum is shown in Figure 2.
The most common bacterial strains employed for bacterial cellulose production are Komagataeibacter, Gluconacetobacter medellinensis strain ID13488, K. intermedius FST213-1, and acetobacter xylinus. Komagataeibacter enzyme converts glucose to gluconic acid and oxidizes the ethanol to acetic acid. The Gluconacetobacter medellinensis strain ID13488 produced a high yield of BC on HS agar plates at pH 4 to 7. K. intermedius FST213-1 enzyme produces maximum BC yield at pH 8–9 [25,26,27].
The bacterial cellulose synthesis is explained in Figure 3 in different segments. (A) One mini-crystal emerges from a single Terminal Complex subunit that assembles the glucose units to form the glucan chain the TCs associate to form a linear TC, which produces bundles of microfibrils. TCs aligned along the longitudinal axis help to propel the cell in the opposite direction to that of the synthesis of the cellulose ribbon. (B). BcsA consists of eight transmembranes (TM) segments and two large cytoplasmic domains, a glycosyltransferase domain in the middle and a C-terminal fragment that contains the domain for activation via c-di-GMP. BcsB has a single TM domain and is in the periplasm. Together, BcsA and BcsB form the catalytic core of cellulose synthase. BcsC spans the outer membrane and the periplasmic space and plays a key role in the crystallization of glucan chains. It has an N-terminal α-helical part formed by several tetratricopeptide repeats (TPR) domains and a C-terminal part that resembles the outer membrane proteins. BcsD is reported to facilitate the transport of glucan chains outside the cell by forming a transmembrane pore. In K. xylinum, the bcs complex is arranged along the longitudinal axis of the cell that aids glucan chains to form crystalline cellulose ribbons as shown in Figure 3.
Cellulosic fibrils are frequently soaked in functional solutions like essential oils and plant extracts, which are trapped in the interior of the BC network. The bountiful hydroxyl groups of the BC network are appropriate to bind these substances and transfer them to human skin in a controlled manner. The hydroxyl groups present on nanofibrils of BC enhance available surface area and use these substances as a precursor for further modifications like esterification, etherification, oxidation, and polymerization, along with some non-covalent attachment of functional molecules. The genetic exploitation of BC-cultivating bacterial strains alters the production and properties of BC. Co-culturing with beneficial microorganisms enhances the BC functionalities. BC production yield can be enhanced by allowing the direct interaction of nanoparticles with BC-cultivating bacterial strains. The in-situ modification of the culture medium influenced BC production [28,29,30].
Bacterial nanocellulose is purified by washing with sodium hydroxide or, for specific uses, treated with gamma radiation [11]. Bacterial cellulose's remarkable mechanical strength comes from the cellulose's linear chains and strong cohesion between macromolecules [31]. BC: PHB composite with very high transparency is prepared by incorporating bacterial cellulose (BC) into a poly (3-hydroxybutyrate) (PHB) matrix. The composite consisted of the homogeneous nano-sized spherulite and nanofibril of PHB and BC with enhanced thermal stability. The PHB/BC nanocomposite is considered for manufacturing display devices, tissue engineering scaffolds, and food packaging [32, 33].
Bacterial cellulose (BC) properties are altered by opting for non-growth-based additives. poly-3-hydroxybutyrate (PHB) in culture media during biosynthesis. PHB is assimilated into the BC fibrils during biosynthesis and enhances the interface between BC and PHB composite matrix [34].
Bacterial cellulose (BC) and polyhydroxybutyrate (PHB) are polymers of microbial origin listed as auspicious biodegradable materials. BC and PHB are added to form a composite material with improved mechanical attributes. The synthesis of BC/PHB composites did not define well and involved many complicated steps. The BC:PHB composite material is biosynthesized by a simplistic Gluconacetobacter xylinus and Ralstoniaeutropha co-culture in situ method. The fraction of PHB in BC:PHB composite is altered from 15.62 to 42.88% by varying R. eutropha inoculums in media. The BC:PHB composite consists BC fibrillar network with connected PHB particles. Bacterial cellulose and cellulose nanocrystals are filled in PHB by solvent casting method, but composite material does not significantly improve PHB properties due to limited dispersion of BC in the PHB-CNC network [35].
The market existence of bacterial cellulose
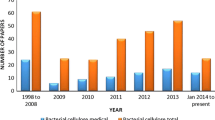
Some reviewers have studied the potential of applying BC in different conventional and industrial products and predicted the future scope of the bacterial nanocellulose market. The last decade has registered remarkable progress in using BC as a drug carrier for controlled delivery, but stagnation could be observed within the last couple of years. Many BC-based products having functional ingredients are in clinical runs or working hard to get market acceptance. In medical textile applications, polyhexamethylenebiguanide (PHMB) containing BC can commercialize as wound dressing with the trade name Suprasorb® X + PHMB [36]. The bacterial nanocellulose is a carrier to hold algae extracts, glycyrrhetinic acid, hyaluronic acid, essential oils, and vitamins for skin wellness effects like bleaching, photodynamic therapy, peeling, and needling. BC's extremely high surface area to volume ratio offers enormous potential to hold different ingredients like a drugs or wellness substances. The worldwide bacterial cellulose market size is anticipated to reach USD 950.7 million in 2028 from USD 296.9 million in 2020 and recorded a compound annual growth rate of 15.8% during the predicted span. The worldwide distribution of research papers on BC indicates the research interests of different nations, which reflects the motivation sign growth in BC-based product development.
Fuctionalization of bacterial cellulose
BC’s molecular and morphological structure supports this unique biomaterial's functionalization. The functionalization of bacterial cellulose has enhanced the applicability of this innovative biomaterial, increasing the wellness of different segments of human life.
Drug loading
Under physiological conditions, the BNC is not biodegradable, which does not assist in drug release. Hence modification of BNC is required to make it more functional.
Drug-loading strategies are selected based on drug molar mass, physic-chemical nature, solubility, drug release profile, stability during each stage, and therapeutic dose. Some BC-related factors, such as fully hydrophilic, semi-dried, freeze-dried, and selected bacteria strains for BC production, influence the drug loading method and potential. Despite considerable variation in loaded drug size, hydrophilicity, lipophilicity, and stability, few drug loading techniques are well applied. Drug loading strategies are classified into two broad classes in situ and post-synthesis. The in-situ drug loading strategy is known for drug addiction during the synthesis of the BC fibril network, but this technique is used at limited places due to the eventual reduction of growth of BC-producing bacterial strain by catalytic ingredients [37]. The post-synthesis drug loading technique is widely adopted by various researchers in which drugs are included in the performed wet fleeces of BNC after purification by the sorption technique. The sorption strategy is performed by gentle stirring for 24 to 48 h under submersed conditions [38].
The larger molar mass and hydrophilic drugs showed a delayed-release profile [39]. An efficient and controlled drug release profile is preferred to transfer the technology commercially.
This method is relatively easy to perform under mild conditions, but due to its sluggish nature, two rapid alternative methods are also tried at industrial fronts, in which one is based on the loading of proteins with BC by the vortex method, which can upload the same amount of protein within ten min as loaded by the conventional method in 24 h. In the case of constant and uniform protein distribution, the fibril network of bacterial cellulose affects the drug release profile [40].
To modify the drug release profile of oral drug BNC-g-poly(acrylic acid) hydrogels as enteric-coated systems are used to release albumin only after increasing the pH to 6.8. In the alternate method, dried and semi-dried bacterial nanocellulose membranes are soaked in a drug solution, which possesses some advantages of a lower amount of drug requirement with a higher potential of drug loading [41]. Different strategies of drug application on BC are shown in Figure 4. BNC is boiled in a drug solution to obtain 30–50% drug loading for controlled drug release [42]. Due to the extensive hydrophilic BC surface area, the post-loading of drugs in the BC network mainly depends on diffusion and capillary forces [43]. Therefore, the successive drug release phenomenon follows the principles of physical transport in which diffusion and swelling concepts are overlapped. The resulting drug-loaded BCs show a biphasic drug release profile in which the first part is an immediate burst release that continues to 0.5–10 h, followed by the second phase of controlled release upto 24 h. This drug release mechanism is appropriate for diclofenac, octenidine, PHMB, benjalkonium, and silk sericin [44]. Some other factors, available surface area, dimensions, drug concentration, the water content of BNC, incubation time, and shaking frequency, also influence the drug loading and release profile of drug-loaded BCs [45].
Prolonged drug release from BNC over weeks and months was achieved by the leading water-insoluble drugs, which need intelligent drug-loading strategies. The innovative drug loading methods consist of chemical modification of BNC, covalently bonding of drugs with BNC fibrils, and application of diffusion or release suppressing chemicals. Due to drug-drug interactions, the π–π clamping increases the NBC payloads and drug release for upto 6 days at a high concentration of doxorubicin when alginate gel was developed inside the BNC scaffolds by electrostatic interaction [46, 50]. Various strategies of drug loading on BNC are illustrated well in Figure 5.
The periodate-oxidized BC has illustrated modified morphology and altered mechanical characteristics of natural BC [47]. The degradation of bacterial cellulose and drug release profile over some time of degradation should be tailored per the targeted application. The micro and nanoencapsulation of lipophilic retinol into poly(ethylene oxide)-b-poly(ε-caprolactone) nanoparticles go after by accumulation in BC fibrils stopped the collapsing of retinol and suppressed the drug delivery concerning about any particle size [48]. Different researchers have opted for hydrogel structures, nanoparticle trapping, the formation of micelles, cyclodextrins, and immobilization on BC surfaces to delay the drug release from BC [49, 50]. The silver and zinc oxide nanoparticles submerged in the BC structure revealed high antimicrobial potential as required for wound dressings, which must change after a prolonged period without damaging regenerated tissues compared with traditional wound treatments [51, 52]. The molecular moderation of bacterial nanocellulose is targeted to engineer the drug release profile by establishing covalent bonding, electrostatic or hydrophobic binding by amidation or phosphorylation, esterification, and etherification [53]. The direct attachment of drug molecules by covalent bonding on BNC is another option to functionalize the BC.
Integrating smart polymers with bacterial nano cellulose (BNC) by blending or grafting offers more functionality with precise control of the release profile. Blending gelatin with BC offers pH-dependent swelling functionality, which may benefit medical and cosmetic applications [54]. Contrast pH-dependent swelling functionality is obtained using a combination of BC-gelatin. BC, poly(vinyl alcohol), and chitosan form a composite film to slow down the delivery of ibuprofen by increasing pH from 1.2 to 7.4 [55]. BNC alginate composites cross-linked by calcium chloride films are used to design binary-guided drug release by electron response and pH. Many challenges await designing a controlled-release drug system for BC’s tumour microenvironment, inflammation, and fever.
The combination of antiseptic octenidine, BC, poloxamer micelles, and gels presented the longest drug-delivery contour over up to eight days with better antimicrobial and mechanical potential properties [43]. More intelligent drug release profiles imparting a well-defined digression from conventional binary phase profile or, in other words, recurrent delivery and gradual profile could benefit stylish applications. The vacuum system is also applied to load drugs in a layered form on BC within a short period. Functional coating and spraying on BC fleece also offer a delayed release of drugs from BC matrices.
The drug-containing microcapsules offer delayed drug release when covalently connected with BC. Encapsulating drugs by a long-chain polymeric compound covalently connected with BC also gives prolonged drug release.
Bacterial cellulose's biomimetic hydrogel fiber (BHF) is developed to mimic lotus fiber in spiral form with high strength, toughness, and energy dissipation potential. Due to high stretchability, porous structure, hydrophilicity, and excellent biocompatibility, the BHF fiber will find enough assistance in tissue regeneration in wound regions [56]. A bio-polymer composite is synthesized by mixing aloe vera (AV) gel 30% v/v in the culture medium throughout the biosynthesis using Acetobacterxylinum in static harvesting. The interaction between aloe vera and bacterial cellulose is proved by FTIR scanning. The AV: BC bio-polymer film presented upgraded crystallinity, mechanical stability, water absorption potential, and water vapour permeability than the entire pure BC film. The modified composite AV: VC film presented fifth times finer pore size than the unmodified film [57].
The role of BC in drug delivery
Absorption, inhalation, swallowing, and intravenous injection are common routes of drug delivery to generate a systemic pharmacological effect. The oral feeding course is the most adopted route of drug delivery to human beings, where the drug is swallowed. However, the poor absorption and biodegradability of high molecular weight peptides and protein drugs give weak membrane permeability [58].
The extraordinarily high biocompatibility of BC in vivo, in vitro, and ex vivo in the long-term application of upto 1 year can outline against various species like mice, pigs, rabbits, rats, and humans without any remarkable toxicity on the genetic or cellular level, fibrosis, and inflammation have proved the suitability of BC in health science [59,60,61]. However, while the database of preclinical in vitro cyto and chemo toxicity experiments in cell cultures and tissues is increasing, comprehensive in vivo studies, especially for defined applications, are still rare. The well-defined applications of BC prove it a highly promising carrier for controlled drug delivery. Varying raw-material, culture conditions, low stability of raw materials, and possible impurities restrict the application of BNC in the medical and pharmaceutical industry.
The bioavailability of drugs on the mucous layer remains for a small duration with a weak diffusion rate that can be maintained using mucoadhesive drugs. The drug release time from the bacterial Nano cellulose network varies from a few minutes to several days. Cellulose and its derivatives have several active groups that can be readily altered to achieve pH sensitivity, target specificity, and sustained action, making them appropriate for targeted drug delivery. To fulfil the need for broad application of controlled drug delivery system, automation and upyielding processes with reproducible potential and consistent quality is highly demanded. Bacterial cellulose production follows traditional homemade fermentation processes and is harvested as fleeces in glass vessels or plastic pans in the cosmetics and food industry [62, 63]. The low aqueous solubility and poor stability of drugs under physiological conditions are significant challenges in successfully formulating controlled drug delivery system development. However, nano-drug release systems exhibit great potential to overcome poor aqueous dissolution and stability. Furthermore, these nano-drugs present the controlled delivery of therapeutic agents on the targeted site.
Numerous biotechnological approaches are invented, ranging from batch processes to continuous harvesting methods. To date, a film like BC fleeces has got commercial acceptance in the drug delivery system. Conventional cultivation is based on the static route in a pan-like reactor, while modern mass-scale production processes opt for the latest biotechnological techniques with uniform fibril networks and high mechanical strength [64]. In addition, stirred harvesting in submerged cultures in agitated aerated fermenters has scaled up the production yield in pellicle form [65]. The production yield of agitating processes remains higher than the static culture process because the shear stress assists the bacterial mutation into non-cellulose-producing strains. An exhaustive research drive is needed in this field to establish more acceptable reasoning. The complete or partial dehydration of BNC is advantageous to enhance packaging, storage, handling, controlled absorption of wound exudates, and reducing the risk of bacterial contamination. The conversion of BNC pellicles into xerogels faces several challenges because mechanical or solvent thermal treatments require altering the 3D network, becoming the cause of the loss of beneficial features of BNC. Freeze-drying, step-wise solvent removal, critical point drying, additional heating, and use of water-absorbing materials are typical de-watering techniques adopted in BNC dehydration [66, 67].
Air-drying of BNC has resulted in terms of 3D structural collapse along with model drug-releasing potential [68].The drug releasing potential of cellulose: collagen composite dressings have proved very effective in the quick recovery of diabetic foot ulcers by promoting angiogenesis with a quick re-epithelisation rate [69]. The addition of curcumin, a natural antimicrobial, reducing agent, and polyphenolic compound are loaded on bacterial cellulose for further enhancement in wound-healing potential, however the hydrophobicity of curcumin restricts its applications in this sector. The overcome the effect of hydrophobicity, the curcumin is microencapsulated by hydroxypropyl-β- cyclodextrin in the form of a hydrogel, which was found effective in wound healing management and illustrated enough biocidal potential against the most common wound affecting S. aureus, Pseudomonas aeruginosa, and Candida Auris pathogenic microbes [70].
However, these hydrogels still have some issues related to homogenization. Hence polyethyleneimine (PEI) is added by a two-step single bath method opting for epichlorohydrin (ECH) as a coupling medium to get suitable hydrogel for antimicrobial applications. The prepared hydrogels show thermal stability, moldability, and PEI-content-dependent mechanical properties. Furthermore, the antibacterial properties of the prepared hydrogels are investigated against S. aureus and E. coli by the agar well-diffusion method and the colony-forming unit method. All hydrogels containing PEI show antibacterial properties dependent on PEI content. The hydrogels containing PEI ≥ 12.88 mg/mL show more than 99% antibacterial activity against both strains, and prepared hydrogels can find potential applications in biomedical fields [71]. A green hydrogel is manufactured by combining two biopolymers, bacterial cellulose and schizophyllan (SPG), after functionalizing BC 3-aminopropyl triethoxysilane (APTES) to create more active sites on the BC network for better integration with SPG. The coupling between BC and SPG is confirmed by X-ray diffraction, thermogravimetric analysis (TGA), and scanning electron microscope (SEM). The manufactured hydrogel has found a remarkable improvement in tensile strength and degree of swelling with moderate antimicrobial functionality [72]. Cationic BC hydrogel is prepared by adding cellulose-bearing imidazolium tosylate functional group (Cell-IMD) and cationic water-soluble cellulose derivative in media during BC synthesis. The inter-fibrillar network of BC with 2,4 and 6% cationic cellulose is synthesized through in situ biosynthesis. The functionalities of cationic BC hydrogel are confirmed by in vitro study with L929 cells in the form of remarkable enhancement in cell proliferation after one week. The cationic BC hydrogel proves adequate antimicrobial strength against S. aureus, S. mutans, and C. albicans [73].
The three-dimensional printing of active biomaterial is highly demanding in tissue engineering. To make 3D printing of polysaccharide-based hydrogel, sodium alginate (SA), 2,2,6,6-tetramethyl piperidinyl-1-oxyl (TEMPO)-oxidized bacterial cellulose (TOBC), and laponitenanoclay (Xls) printing ink is used. The printed structure has ensured the delayed release of protein to be used for biomedical applications, controlled drug delivery, and tissue engineering [74]. A compound hydrogel is manufactured by opting for 1%, 1,4-butanediol diglycidyl ether (BDDE) as a crosslinking agent to crosslink BC and 2% hyaluronic acid (HA). Networked BC/HA composite materials show a denser and smoother surface than pure BC surface, which improves water retention potential and dimensional stability. BDDE:BC:HA composite exhibits a tensile strength ∼ of 0.61 MPa, maximum thermal degradation at 360 °C, and has many applications in wound care management [75]. The enzymatic hydrolysate (EH) dosed moso bamboo (MBEH) is a carbon source to generate sodium alginate bamboo bacterial cellulose. The sodium alginate is added from 0.25 to 1.0% to enhance thermal stability. The composite shows a pH-dependent dynamic swelling feature: 613% at pH 7.4 and 366% at pH 1.2, owing to electrostatic repulsion of –COO–. Protein-based drug bovine serum albumin is illustrated by controlled release from MBEH-SA-BC nanocomposite hydrogel [76].
A second-generation broad-spectrum antibiotic doxycycline 0.14 mol/L is loaded with oxidized BC and has a drug delivery of < 10% after the eighth day of its application at 25 °C. The doxycycline is loaded to BC and has good biocompatibility in the in-vitro toxicity test (MTT assay). The agar diffusion test proves the antimicrobial potential of modified BC against oral pathogenic microbes. The drug-loaded BC is found appropriate for dental extraction [77]. Oven-dried and freeze-dried BC is used to load the drug diclofenac sodium. The freeze-dried drug-loaded BC exhibits an instant first-order release, while the oven-dried BC shows a prolonged non-Fickian second-order release for different types of pain management. Polyethylenimine grafted BC aerogel is developed by loading aspirin and gentamicin for a pH-sensitive controlled drug delivery system and characterize for cytotoxicity [78]. Cloxacillin (CLX) and cefuroxime (CEF) sodium salts loaded BC is prepared by injection moulding method and shows the best results at 1% drug loading in microparticulate form. The modified BC does not show effective prolonged drug release, and 85% of the drug is released within 30 min [79]. The in-situ BC network modification is performed by incorporating water-soluble poly (ethylene glycol) 400 and 4000 into BC. The drug diclofenac sodium anti-inflammatory model is loaded on a modified BC network by spray technique and found quite efficient. Nitrogen dioxide is opted to produce carboxylated BC in chloroform/cyclohexane by oxidation and is engaged as a carrier for controlled delivery of the antitumor substance cisplatin (CDDP). The nitrogen dioxide is established to penetrate BC crystallites and enhances the available carboxyl group upto 4 mmol/g. The immobilized CDDP has proved the prolonged release in place of instant burst and opened new frontiers for regulated drug delivery systems [80]. For therapeutic applications, homogeneous and permeable BC Diclofenac sodium-loaded membranes are produced [45]. The defibrillation of bacterial cellulose nanofibres (BCNF) also remains helpful for better drug-holding. Bacterial cellulose nanofibres (BCNF) are defibrillated by ultra-refining and spray drying with mannitol, maltodextrin, or hydroxypropylmethylcellulose. BCNF is loaded with diclofenac sodium or caffeine. The composite BCNF fibres exhibited controlled pH-sensitive gastro-resistance drug delivery. This concept opens new opportunities to load new biomaterials for controlled release [81]. Tetracycline hydrochloride: BC composites were detected with excellent antimicrobial potential with zero cytotoxicity in HEK293 cells, which is essential for wound dressing applications [34]. BC loaded with octenidine creates active wound dressing because octenidine release becomes possible after swelling and diffusion [40].
Zinc, a metal, can give antimicrobial potential cheaper than silver. BC/ZnO nanocomposite membranes show antimicrobial activity against Escherichia coli (E Coli), Pseudomonas aeruginosa, Staphylococcus aureus (S. aureus), and Citrobacterfreundii with 66% healing after 15 days [56]. Vaccarin-impregnated BC alters the subcutaneous tissue formation of collagen fibers and hyperplastic fibrous connective tissue [67]. The lidocaine hydrochloride and ibuprofen loading with BC possess uniform distribution of both drugs in the BC membranes. Ibuprofen's three times slower permeation than lidocaine hydrochloride makes it an effective drug delivery system [82]. The BNC, loaded with polihexanide and povidone-iodine, is given a prolonged antiseptic effect by diffusion and swelling. Povidone-iodine (PI) loaded BC illustrates a controlled release compared to PHMB and high compatibility with human keratinocytes due to high molar drug mass and structural changes induced by PI insertion into BC [39]. Biocompatible and mucoadhesive bacterial cellulose-g-poly (acrylic acid) hydrogels loaded with bovine serum albumin (BSA) provide controlled BSA release in the stomach's acidic environment. The cumulative release of less than 10% of BSA in simulated gastric fluid (SGF) is achieved with structural integrity and bioactivity in hydrogel forms. BC is mixed with octenidin:poloxamer hybrid system to form a membrane and achieve a long-term controlled release of octenidine to assist in the advanced treatment of infected wounds with improved mechanical and antimicrobial properties [43].
Bacterial cellulose: ibuprofen: sodium alginate dual stimuli functional composites are synthesized to achieve pH and electric field stimulus-responsive swelling properties and the in vitro stimulus-responsive drug release. The ibuprofen release is controlled by deprotonation or protonation of calcium alginate in the hydrogels under different pH conditions. The drug release is faster in alkaline conditions and slower in acidic conditions, in which drug release is enhanced by electric stimuli [83].
The polyvinyl alcohol is mixed with nano-fibrillated BC and nano-powdered BC to cast biodegradable and biocompatible composites. The composite membranes show drug diffusion, mass transfer potential, solubility, swelling, and moisture vapour transmission. Tetracycline hydrochloride, the drug, is used to test the controlled release through this composite membrane. The drug release model is developed by considering experimental and environmental data. The membrane's composition, number of layers in the composite, and thickness are found to be practical attributes in regulating the drug release profile through the composite. The BC nanofibril’s thickness evinces the deciding aspect of the drug release study. The controlled drug delivery is registered for several days in the case of multi-membrane composites [84]
High-speed drug loading to BNC offers retarded protein delivery as per network density [85]. The laccase immobilization on BNC provides flexibility and accessibility of the drug to be worked against gram-positive and gram-negative bacteria [86, 87].
BC membranes are found supportive for the dermal release of lidocaine for local anaesthetics to the human epidermal cell at a delayed rate [88]. BNC is functionalized by arginine, glycine, aspartic acid, cysteine, and gentamicin by grafting assistance of coupling agent 3-aminopropyltriethoxysilane. The covalent attachment of gentamicin onto the BC network is created, which becomes effective against S. mutans, with nontoxic to human dermal fibroblast, and is useful for wound healing and drug delivery system [89].
The BC is effective against Staphylococcus aureus after saturation with antibiotic fusidic acid for wound healing. BNC transfers 1,3-dihydroxy-2-propanone in the corneum extract to alter skin colour [90]. BC:Chitin is also tried to form bacterial nanocellulose: regenerated chitin fibres (BCNC: RCF) biocompatible sutures, which are found effective in wound management. The enzymatic degradation rate is also controlled by varying concentrations of BCNCs in the sutures, while chitin content is proven effective in promoting in vivo cell proliferation on surfaces, which is found effective in wound management [91]. The antimicrobial potential of BC is also enhanced by modifying the BC with collagen deposition to promote fibroblast infiltration and new collagen deposition in the wound bed through enhanced cell alignment [92].
Polycapralactone is introduced into the bacterial cellulose network to incorporate durable flexibility in the BC:PCL membrane. The BC:PCL membranes are additionally functionalized to create wound dressing composite scaffolds with two antibiotics, gentamicin and streptomycin, to assist in wound healing. FTIR confirms the presence of drugs in BC:PCL composite membranes. The dispersive energy X-ray (EDX) BC:PCL composite confirms the presence of sulfur with superior antimicrobial properties against E. coli and S. aureus bacteria. The release profile of antibiotics from composite shows burst release (42%) in the initial 6 h, followed by a controlled release for the next 48 h (52%) [93].
Cassava waste water (Manihotesculenta) is a carbon source for BC biosynthesis. The silver nanoparticles are impregnated with BC, followed by a NaBH controlled reduction process to form an Ag:BC composite. The Ag:BC composite consists of fibrils of less than 100 nm width. The composite is found to be effective in controlling the bacterial growth of Escherichia coli, Bacillus subtilis (BS), Staphylococcus aureus (SA), Salmonella typhi (ST), and Pseudomonas aeruginosa (PA) [94]
The BC and CMC blends are impregnated with turmeric extract and citric acid. Citric acid is used as a crosslinking agent to enhance composite stability and delay drug release. The composites exhibit remarkable swelling potential, which is beneficial for absorbing wound exudates. The composite shows sufficient antimicrobial potential against E. coli, S. aureus), and Candida albicans (C. albicans) [95].
The very high phenolic content of Epilobium angustifolium L (EAL) makes this plant extract very useful for antimicrobial, anti-inflammatory, anticancer, and antioxidant applications. The ability of ethanolic extract EAL to stop cell growth is tested on normal human fibroblasts (HDF) and cancer cell lines from the breast (MCF7), colon (HT-29), lung (A549), liver (Hep G2), and melanoma (A375). The bacterial cellulose is used as an encapsulating shell for the delayed release of plant extract (EAL). Finally, the BC:EAL potential as an anticancer agent is examined against the HT-29 cell line, which exhibits maximum responsiveness to the EAL extract. The biocompatibility of pure BC and EAL doses and the time-dependent cytotoxicity of EAL delivery are validated and found to be sufficient. The plant extract leaches out 2.5% EAL of BC, remarkably suppressing cell viability to 18.16% and 6.16% of the controlled values, respectively. The enhanced number of apoptotic/dead cells up to 37.53% and 66.90% after 48 and 72 h of treatment, respectively, concludes that the BC shells are suitable to be used as an anticancer drug carrier and deliver it in a controlled fashion in the targeted zone [96].
BC:TiO2 nanocomposite shows antimicrobial activity against E. coli and S. aureus bacteria during wound healing [97]. BC/ε-poly-l-Lysine (ε-PLL) nanocomposites also exhibit antimicrobial activity, securing beneficial BC structural and mechanical features. The modification with nontoxic biopolymer ε-PLL restricts the growth of S. epidermidis on the membrane surfaces [98]. A sustained release of Ag + from BC: Ag nanoparticle composite prolongs antibacterial potential against S. aureus [99, 100].
Silymarin (SMN)-zein nanoparticle: BC nanocomposite shows lipid oxidation, wettability and swelling change, and antioxidant and antibacterial activity [101].
BC/carboxymethyl cellulose:methotrexate composites are found to be effective for antimicrobial properties against gram-positive and gram-negative bacteria [102]. BC:PHEMA hydrogels are synthesized by in-situ ultraviolet (UV) radical polymerization, which enhances the tensile strength, wound healing, nontoxicity, proliferation, and possibilities of tissue replacement [103]. BC: polyvinyl alcohol and BC: Hyaluronic acid composites are helpful in the creation of artificial corneas due to their ability to enhance the visible light transmittance [104].
BC: Iron oxide nanoparticle-based magnetic domains are introduced to achieve with required Young’s modulus for blood vessels [105]. BC with tuned porosity exhibits larger pore size than native BC to allow muscle cell in-growth [106]. BC: urinary bladder matrix proves higher adhesion and proliferation of retinal pigment epithelium cells than uncoated BC and closer recapitulation of the in vivo cell phenotype than uncoated BC [107]. Nisin is immobilized on BC by crosslinking between the amine groups of nisin with the C6 carboxylic acid group of bacterial cellulose. BC: Nisin biomembranes are found to be effective antimicrobial surfaces against gram-positive and gram-negative bacteria [108, 109].
BC tubes are impregnated in ε-polylysine (ε-PL) and produce active sausage casings, which have good tensile and barrier strength and ample antimicrobial properties with extended self-life of the sausage [110].
Good biocompatibility, biodegradability, highest possibilities of modification and low immunogenicity of natural polysaccharides such as BC is observed as an appropriate biomaterial for controlled drug delivery system. As a drug nanocarrier, the BC can be used for diagnostics, drugs, and therapeutics. The bacterial cellulose can hold nanoemulsions, nanohydrogels, nanoliposomes, and nano micelles for diabetes management.
Due to its biocompatibility, biodegradability, bacterial nano-cellulose composite formation, and versatile surface chemistry, the BC may explore some exciting possibilities as a drug delivery carrier. Some latest methods of functionalization of BC may appear in future due to the day-by-day increasing demand for inventions in the controlled drug delivery segment. Although, the most popular drug loading technique in BC structure is the immersion of BC in drug solution, generally followed by lyophilization to achieve maximum drug add-on on the BC membrane. The popular drugs to be uploaded into BC fibrillar network are diclofenac and ibuprofen as anti-inflammatory medications and antimicrobial drugs for microbial control. For cancer treatment, BC is shined as a reliable biomaterial. BNC-containing scaffolds popularly opt to fight against cancer. Graphene oxide is also finding its way to functionalize the BC for the potential generation of controlled release of various anti-inflammatory drugs. BC-based hydrogels will also play a decisive role in oral protein delivery.
Role of BC in medical textiles
The outstanding properties of BC push it to be a promising material for preparing various implantable and non-implantable medical textiles.
When selecting a biomaterial for biomedicinal applications, biocompatibility, toxic effect, and immunological and physiological reactions are the significant attributes to be considered. These biomaterials did not have a three-dimensional porous structure, which remains beneficial to hold functional materials for biomedicinal applications [111, 112]. The three-dimensional porous network of dressing material remains beneficial for wound healing management due to its ample potential to transfer antibiotic drugs or other medicines into the wound and act as a skin layer to protect from secondary infections from another microorganism [113]. The bacterial cellulose has an appropriate fibrillar network, but due to the absence of antimicrobial potential, it must load other biologically active molecules with much functionality [114].
Two functional agents, glycidyltrimethylammonium chloride and glycidylhexadecyl ether, are opted for the covalent formation with glucose hydroxyl groups through a heterogeneous reaction in alkaline pH conditions. The BC composite adequately reduces the ~ 50% growth of S. aureus, subsp. aureus Rosenbach 6538PTM and E. coli (Migula) Castellani and Chalmers. The modified BC hydrogels proved cell viability during wound care in the 90 to 100% range [115]. BC is synthesized on cotton gauze to enhance water absorbency and wickability for effective wound management [116].
Multifunctional bactericidal agents are needed to face the global danger of antibiotic-resistant bacteria. Silver-containing metal–organic framework (SOF) nanoparticles are mixed with nano-sized bacterial cellulose and chitosan slurry to make an antibacterial porous fibrillar network that looks like the extracellular matrix of the skin. This is done to fight against bacteria that are resistant to antibiotics. The SEM images confirm the existence of 30 nm fibrils in the composite. The (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay shows a biocompatibility of 94% with cell viability for bacterial cellulose: chitosan composites. These biocomposites prove sufficient antimicrobial potential against E. coli and S. aureus bacterial strains. The BC: CS:25%SOF composite membranes show outstanding healing performance with 74% wound healing and tissue generation after 10 days of surgery during animal in-vivo studies [117] Fortune Minerals Ltd. London developed a wound care system applicable to cover human skin burn areas with the name of ‘Bio Fill’, and a similar product was developed by Biofill Products, Ltd Hungary with a trading name of ‘Bioprocess’. Jenpolymer Materials UG & Co. Germany evolved a tubular implant for coronary artery bypass surgery with a commercial name of ‘Basyc’. CP Kelco, Mumbai, India, developed a suspension of encapsulated enzymes/particles with the commercial identity of ‘Cellulon PX microfibrous cellulose’. CP Kelco extended its product profile by developing the nonwoven structure as a binder with the trade name of ‘Cellulon’. The government of Polland flourished Protective jackets and dressings to offer protection to minors from burn possibilities with the label ‘CelMat (C) MG &CelMat (R) MG’ [118]. Xylos corporation, USA, matured the invention of the biocompatible implant containing BC as an essential drug carrier to treat injuries and wounds and added a tissue reinforcement matrix for tendon repair with the commercial name of ‘Securin’ [119]. Blood vessels carry the oxygen holding blood all-round the human body before delivering back to the human heart. Fine-diameter blood vessels (100 mm length and 4–5 mm inner diameter) with a 3D fibrillar structure from bio-designed BC cellulose are manufactured using gluconacetobacter strains and matrix reservoir technology. BC tubes are used as a scaffold to replace the carotid arteries and check in-vivo performance [120]. A novel G-BNC bioreactor consists of a central axial glass rod placed within a silicone tube to manufacture a G-BNC conduit. The physicochemical characteristics of the BNC tubes fabricated in three different bioreactors to be used for an ePTFE vascular prosthesis in medical applications [121]. Vascular prosthesis is a technique to redevelop the artificial circulatory system in the body, including blood. BNC and thyme, astragalus, and Ziziphus honey composites are used to assess the antimicrobial, permeability, and Wettability potential. Thyme honey: BNC composites have shown the highest antimicrobial potential [122]. The calcium phosphate is integrated with BC in hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) in various proportions from 00:00 to 60:40 for scaffold manufacturing. The scaffold preforms are manufactured after being included with polyvinylpyrrolidone (PVP), poly(ethylene glycol), agar, and glycerin. These scaffolds have shown enough viscoelastic potential between 28 and 37 °C. These scaffolds are found suitable for bone tissue regeneration [123]. The methacrylate gelatin (GelMA): bacterial cellulose compound materials are formed after sinking BC films in GelMA solution followed by photo-guided crosslinking. These hydrogels have excellent interconnection in the fibrillar network with reduced average pore size from 200 to 10 µm with increased available surface area and BC share. BSN Medical Gmbh adopted this concept and manufactured wound dressing consisting of a BC layer with a bacterial-absorbing design [124]. The GelMA: BC composite has good biocompatibility with human knee articular chondrocytes. The human cells are encapsulated inside the GelMA: BC composite hydrogel to keep the chondrocyte phenotype active for seven days for cartilage tissue regeneration [125].
The bacterial cellulose coating is performed through conventional dip-nip coating in a suspension containing cellulose-producing bacteria. As the bacterial cellulose-producing bacteria adheres to the objective surface, bacteria initiate the secretion of bacterial cellulose on the objective surface as a porous 3D hydrogel, which is helpful for biomedical applications [126]. The bacterial cellulose is used to scavenge silk fibroin to create a double network to hold more functional molecules inside the 3D network of BC to be used for cartilage tissue regeneration [127]. The system can be used as an effective drug carrier due to the availability of the double network. Hybrid alginate: casein hydrogels are impregnated with bacterial cellulose with iron-oxide nanoparticles. The iron-oxide nanoparticles and the ionic crosslinking among the calcium ions, alginate, and bacterial cellulose film (BCF) enhance the stability with appropriate porosity. Alginate provides controlled gelation, biocompatibility, and bacterial cellulose reinforcement to form tunable alginate-casein-bacterial cellulose (ACB) composite hydrogel. The ACB hydrogel is suitable for cell adhesion by extra pore roughness, high water uptake, and thermal stability for effective wound dressing. The delayed release of the drug makes it an appropriate sustainable material for innovative wound dressings. These gels are found favourable for injectable purposes for transdermal drug delivery [62, 128]. The head-on toppling of anionic polyelectrolyte carboxymethyl cellulose (CMC) into bacterial cellulose (BC) matrix is to fabricate the biocompatible and re-swellable hydrogel to be used as a colourimetric sensor. It can differentiate different colours as changes as a function of pH, which remains difficult to catch by the naked eye. This bio-sensor has found its application in sweat Ph determination with high accuracy, which can be used to predict on-skin health status [129]. The polyacrylamide/iota-carrageenan hydrogels are synthesized through a one-bath radical polymerization and strengthened by introducing bacterial cellulose crystals, forming the intermolecular hydrogen bonds and surface interconnect between microclusters and polymer network. These hydrogels can withstand over 200 kPa of tensile stress, reach a toughness of ∼2000 kJ/m3, and present a water-sensitive shape memory effect by a recovery ratio of 84.3% in 4 min [130]. BC polymer is in situ modified by crosslinking agent citric acid in the presence of catalysts disodium phosphate and sodium bicarbonate to enhance water holding capacity 1.5 times higher than traditional wound dressings to treat exuding wounds highly without any cytotoxicity against in vitro fibroblast cell line L929 [131].
BC in cosmetics
The ever-increasing desire for beauty across the globe is responsible for exploring state-of-the-art cosmetic initiatives and taking them to the skin to cause penetration for wellness. Natural materials like cellulose nanofibres, cellulose pulp, and hydrogels have been utilized as essential materials to convert them into cosmetic ingredients. The bacterial nanocellulose (BNC) is a 3D network of nanofibres with an average diameter of 70 nm, smaller than human skin pore size. Consequently, the fine BC nanofibres have penetrated the human skin to carry various functional ingredients. Various facial mask manufacturing companies have utilized this feature of BC in the cosmetic market. Bacterial cellulose, researched as matrices for controlled drug release, has motivated cosmetic scientists to deliver hydrophilic and hydrophobic cosmetic ingredients to deliver on human skin [10, 132, 133].
BNC: caffeine composite offers a topical delivery mechanism with delayed permeation of caffeine compared to traditional aqueous solutions and gels to create cellulite-treating patches [134]. Cholinium-based ionic liquids paired with anions made from phenolic acids like caffeic, ellagic, and gallic and incorporated into BNC membranes showed increased rehydration ability, slow and sustained release, and antioxidant and anti-inflammatory activities [135]. In another similar work, cholinium-based ionic liquids are paired with vitamin B anions and uploaded with BNC membranes, which enhance rehydration potential, reduce brittleness, and facilitate the fast release of active substances [136]. Poly(ethylene glycol diacrylate) 5% and BNC 3% are used to form composite films of better viscoelastic properties, comparable to BNC gels for face mask applications. The polyvinyl alcohol: BNC composites are freeze-dried to reduce the possibility of contamination. The lightweight composite film is suitable for facial mask application [137, 138]. A research group has developed a non-invasive protocol for in vivo evaluation of the effectiveness and acceptance of BNC facial masks as a delivery system of active substances for anti-ageing, skin lifting, and skin cell regeneration [139]. The anti-ageing and skin cleansing formulations are prepared by immobilizing papain enzyme covalently on BC surfaces to create an enzyme-based skincare formulation [140]. Individualized cellulose nanofibrils are prepared by oxidizing bacterial cellulose in a tetramethylpiperidine-1-oxyl system. Oxidized BNC nanofibrils produce better oil stabilization (over 8 months): water emulsion interface, which becomes the base for many cosmetic products [141]. The emulsion stabilizer is produced by mixing carboxymethyl cellulose (CMC) with BNC to prolong the stability of skin care products for up to 90 days [142]. Skin moisturization is an essential part of beauty care. The glycerin-containing BNC films show a high skin moisturizing effect with low skin irritation and tolerance [61]. Soothing neem leaf, conditioning shell ginger, hydrating amino acids, and vitamin C are added to BC to form a bio-cellulose mask to ensure a moisturizing, cleansing, and glowing skin effect [62].
Scrubbers are widely used in cosmetic and body care activities, and bacterial cellulose can be explored in this segment due to the significant release of microplastics from conventional scrubbers. The BC scrubber is manufactured by incorporating various proportions of NaOH-PEG and flavonoids with BC to optimize its antimicrobial and cleansing potential. The modified BC scrubbers prove to have better antimicrobial potential than traditional scrubbers. SEM–EDX and FTIR analytical techniques proved the three-dimensional network and the presence of flavonoid particles [143]. The healthcare potential of kombucha is integrated with BC (KBC) for biomedicinal applications. KBC membrane is integrated with silk nonwoven fabric, followed by impregnation in a silk sericin bath for biomedical uses. Various analytical techniques like SEM have proved KBC's presence within a fibrous network of silk nonwoven fabrics. The treated silk nonwoven demonstrated excellent UV protection. The cytotoxicity and (2,2-Diphenyl-1-picrylhydrazl) (DPPH) radical scavenging analysis proved the biocompatibility and antioxidant potential to make it appropriate for skin care, wound healing, and cosmetic purposes [144]. The controlled release of α-arbutin is achieved after immobilizing its bacterial cellulose nanofibrillar pad. Two different methods are used to functionalize the BC nanofibril pad. In the first method, the BC nanofibril pad is dipped in the α-arbutin bath, where citric acid is used as a cross-linking agent. In the second method, the α-arbutin is trapped on a BC nanofibril pad with Tragacanth gum and then stabilized with the ultrasonic-assisted micro-emulsion. This modified BC is found suitable for skin lightening [145]. Glucanacetobacter hansenii, a bacterial strain, can be used to cultivate culture made out of juice from tropical fruit residue as an alternative for the traditional media to get the high nutrient content, mineral content, and enzymatic and non-enzymatic sites in tropical fruits provide the suppression of free radicals of the human skin. The tropical fruit-based BNC facemask is also suitable for skin moisture management [19]. The traditional cosmetic creams consist of 5.5% surfactants to stabilize oil–water emulsions and are replaced by a 0.5% mixture of bacterial cellulose:carboxymethyl cellulose to make it surfactant-free. The demand for surfactant-free cosmetic creams is increasing [146]. The bacterial cellulose has enough attributes such as a three-dimensional fibrillar network with nano-size pores, high purity, moisturizing potential, good mechanical strength with 70–90% crystallinity, high water uptake, biodegradability, and easy functionalization to play a crucial role in the cosmetic industry. The cosmetic industry is also passing through an era of revolutionary changes by introducing nanoparticles, natural polymers, synthetic polymers, and carbon materials to prioritize safety. Shortly, BC-based composite membranes may also find more promising applications in the cosmetic field. Scientists in the global arena are trying to utilize BC for human skin moisturization, skin oiliness management, delayed release of wellness substances such as vitamin E and essential oils, and biocompatibility with human tissues in composite form because bacterial cellulose is a suitable carrier for many active substances. Cosmetic products created with bacterial cellulose through a sustainable development process will replace unhealthy and non-environment-friendly cosmetic products in future. The above discussion will give the roadmap for scientists for future product development.
Bacterial cellulose in biocomposites
BC owns three-dimensional porous aggregates of fine fibrils. It acts as a matrix for housing a variety of particles from different reinforcement materials. The anchored reinforcement materials provide additional properties to BC that impart their natural biological and physicochemical properties. Bacterial cellulose has created its place as an effective matrices material in various biocomposites to hold a variety of active biomaterials for intelligent applications. Strength and stability are two major concerns in hydrogels from the application’s point of view. The BC:polyacrylamide:iota-carrageenan double-network hydrogels were synthesized and followed by tension-drying and post-annealing treatments to achieve tensile stress200 kPa, strain 27%, toughness∼2000 kJ/m3 [83]. Wound healing composite material was engineered by adding BC pellicle into the polylactic acid matrix. The composites revealed enhanced biocompatibility and mechanical strength [55]. TiO2 nanoparticles bonded to the surface of the hydrophilic BC fibrils to enhance the electrical conductivity to be used as a biosensor. The hydrogen bonding forms a fibril network between TiO2 and BC [147]. BC: carboxymethyl cellulose nanocomposites were produced in situ with enhanced viscosity of culture media to reduce the elastic modulus and porosity to be used as a delayed drug delivery system for skin treatment purposes [148].
Magnetic nanoparticle Fe3O4 immobilized on BNC by conducting polypyrrole (PPy) to develop as an electrode [64].
Conclusion
The commercialization of biosynthesis of BC is attainable by exploring the economically feasible BC synthesis techniques to compete with conventional cellulose for cheap product development, contrasting to higher-value biomedical applications. The scope of upcoming technologies to reduce the BC production cost in various cultural conditions uses waste materials from different industries and processes. BC’s in vivo and in vitro medical applications are still in the neonatal stage and require better quality control with high preciseness in BC production.
A promising future for innovative biomedical applications like controlled drug delivery, burn skin management, wound dressing, and scaffolding for tissue engineering based on BC will come from their adaptability in granting new functions across their ease of modification and potential of manufacturing composites. In the advancing juncture, enormous advancement can be made toward the apprehension of interconnection between BC and various cosmetics and drugs, and countless physical and chemical attempts have been registered to manage controlled drug delivery. However, more advanced delivery profiles like H-sensitive effects, multiphase, and pulse are still quite open to being researched for BC applications. Similar challenges are available for more innovations in BC for quicker, controllable, and precise drug and other ingredient loading techniques. The primary threat is insufficient proof of concepts OF BC’s in vitro and in vivo biological uses for critical diseases. Thus, BC's factual application research lacks enough space for progressive ideas concerning loading, stabilizing, and delivering to nurture the succeeding application as a controlled drug and wellness ingredient release system. BC being a nanosized fibrillar network, has drawn immense recognition in the last decade over the last 10 years as a controlled drug-delivery and cosmetic carrier. The unique supramolecular nanostructure and enormous functionalities have guided to sizeable interests in BC-based drug release functionality preferentially for oral administrations, local, transdermal, and or implants. Recent progress in BC cultivation techniques provides opportunities for high-quality and broader application of BC in cosmetics wellness and pharmaceutical products. BC’s natural three-dimensional fibrillar network, proficient in facilitating cell adhesion, will remain attractive to observe progress in this direction by precisely controlling the porosity. Wound healing and skin regeneration are other areas of interest in which BC will make remarkable progress. The application of BC in cosmetic products has opened new frontiers to designing more intelligent cosmetics. Bacterial cellulose can fascinate the world by finding new cosmetics, controlled drug delivery systems, and medical management applications.
References
D. Klemm, F. Kramer, S. Moritz, T. Lindstrom, A. Ankerfors, D. Gray, A. Dorris, Angew. Chem. Int. Ed. 50, 5438 (2011)
M. Shoda, Y. Sugano, Biotechnol. Bioproc. Eng. 10, 1 (2005)
N. Duran, A.P. Lemes, A.B. Seabra, Recent. Pat. Nanotechnol. 6, 16 (2012)
K. Schlufter, H.P. Schmauder, S. Dorn, T. Heinze, Macromol. Rapid Commun. 27, 1670 (2006)
H.S. Barud, C.A. Ribeiro, M.S. Crespi, J. Therm. Anal. Calorim. 87, 815 (2007)
N. Lin, A. Dufresne, Eur. Polym. J. 59, 302 (2014)
R.K. Mishra, A. Sabu, S.K. Tiwari, J. Saudi Chem. Soc. 22, 949 (2018)
P. Phanthong, P. Reubroycharoen, X. Hao, G. Xu, A. Abudula, G. Guan, Carbon Resour. Convers. 1, 32 (2018)
I.A.N. Donini, D.T.B. Salvi, F.K. Fukumoto, W.R. Lustri, H.S. Barud, R. Marchetto, Y. Messaddeq, S.J.L. Ribeiro, Eclét. Quím. 4, 165 (2010B)
G. Pacheco, C.V. de Mello, B.G. Chiari-Andréo, V.L.B. Isaac, S.J.L. Ribeiro, E. Pecoraro, E. Trovatti, Cosmet. Dermatol. 17, 840 (2018)
R. Portela, C.R. Leal, P.L. Almeida, R.G. Sobral, Microb. Biotechnol. 12, 586 (2019)
L.K. Pandey, C. Saxena, V. Dubey, Sep. Purif. Technol. 42, 213 (2005)
G. Shanshan, J. Wang, Z. Jin, Carbohydr. Polym. 87, 1020 (2012)
G. Guhados, W. Wan, J.L. Hutter, Langmuir 21, 6642 (2005)
Y.C. Hsieh, H. Yano, M. Nogi, S.J. Eichhorn, Cellulose 15, 507 (2008)
H. Bodin, H. Backdahl, L. Fink, B. Gustafsson, P.R. Gatenholm, Biotechnol. Bioeng. 97, 425 (2007)
G. Buldum, A. Bismarck, A. Mantalaris, Bioprocess. Biosyst. Eng. 41, 265 (2018)
H.C. Wong, A.L. Fear, R.D. Calhoon, G.H. Eichinger, R. Mayer, D. Amikam, Proc. Natl. Acad. Sci. 87, 8130 (1990)
R. Standal, T.G. Iversen, D.H. Coucheron, E. Fjaervik, J.M. Blatny, S. Valla, J. Bacteriol. 176, 665 (1994)
S.S. Wang, Y.H. Han, J.L. Chen, D.C. Zhang, X.X. Shi, Y.X. Ye, D.L. Chen, M. Li, Polymers 10, 963 (2018)
D. Mikkelsen, B.M. Flanagan, G. Dykes, M. Gidley, J. Appl. Microbiol. 107, 576 (2009)
K. Mehta, S. Pfeffer, R.M. Brown, Cellulose 22, 119 (2015)
J.W. Hwang, Y.K. Yang, J.K. Hwang, Y.R. Pyun, Y.S. Kim, J. Biosci. Bioeng. 88, 183 (1999)
M.U. Islam, M.W. Ullah, S. Khan, N. Shah, J.K. Park, Int. J. Biol. Macromol. 102, 1166 (2017)
S.P. Lin, I. LoiraCalvar, J.M. Catchmark, J.R. Liu, A. Demirci, K.C. Cheng, Cellulose 20, 2191 (2013)
L. Urbina, A.M. Hernandez-Arriaga, A. Eceiza, N. Gabilondo, M.A. Corcuera, M.A. Prieto, Cellulose 24, 2071 (2017)
S.P. Lin, Y.H. Huang, K.D. Hsu, Y.J. Lai, Y.K. Chen, K.C. Cheng, Carbohydr. Polym. 151, 827 (2016)
B. Sun, Q. Hou, Z. Liu, Y. Ni, Cellulose 22, 1135 (2015)
A. Salam, L.A. Lucia, H. Jameel, Cellulose 22, 397 (2015)
U.D. Hemraz, K.A. Campbell, J.S. Burdick, K. Ckless, Y. Boluk, R. Sunasee, Biomacromol 16, 319 (2015)
H.S. Barud, J.L. Souza, D.B. Santos, M.S. Crespi, C.A. Ribeiro, Y.M. Sidney, J.L. Ribeiro, Carbohydr. Polym. 83, 1279 (2011)
C. Zhijiang, Y. Guang, Mater. Lett. 65, 182 (2011)
D.R. Ruka, G.P. Simon, K.M. Dean, Carbohydr. Polym. 92, 1717 (2013)
R. Ding, S. Hu, M. Xu, Q. Hu, S. Jiang, K. Xu, P.L. Tremblay, T. Zhang, Carbohydr. Polym. 252, 117137 (2021)
I.T. Seoane, P. Cerrutti, A. Vazquez, L.B. Manfredi, V.P. Cyras, J. Polym. Environ. 25, 586 (2017)
M.L. Cacicedo, K. Cesca, V.E. Bosio, L.M. Porto, J. Appl. Biomed. 13, 239 (2015)
W. Shao, H. Liu, S. Wang, Carbohydr. Polym. 145, 114 (2016)
C. Wiegand, S. Moritz, N. Hessler, J. Mater. Sci. Mater. Med. 26, 245 (2015)
A. Müller, F. Wesarg, N. Hessler, F.A. Müller, D. Kralisch, D. Fischer, Carbohydr. Polym. 106, 410 (2014)
N. Ahmad, M.C.I.M. Amin, S.M. Mahali, I. Ismail, V.T.G. Chuang, Mol. Pharm. 11, 4130 (2014)
L. Huang, X. Chen, T.X. Nguyen, H. Tang, L. Zhang, G. Yang, J. Mater. Chem. B 1, 2976 (2013)
Y. Alkhatib, M. Dewaldt, S. Moritz, R. Nitzsche, D. Kralisch, D. Fischer, Eur. J. Pharm. Biopharm. 112, 164 (2017)
S. Moritz, C. Wiegand, F. Wesarg, Int. J. Pharm. 471, 45 (2014)
N.H.C.S. Silva, A.F. Rodrigues, I.F. Almeida, P.C. Costa, C. Rosado, C.P. Neto, A.J. Silvestre, C.S. Freire, Carbohydr. Polym. 106, 264 (2014)
M.L. Cacicedo, I.E. León, J.S. Gonzalez, L.M. Porto, V.A. Alvarez, G.R. Castro, Colloid Surf. 140, 421 (2016)
J. Li, Y. Wan, L. Li, H. Liang, J. Wang, Mater. Sci. Eng. 29, 1635 (2009)
Y. Numata, L. Mazzarino, R. Borsali, Int. J. Pharm. 486, 217 (2015)
H. Luo, H. Ao, G. Li, Curr. Appl. Phys. 17, 249 (2017)
S. Sukhtezari, H. Almasi, S. Pirsa, M. Zandi, M. Pirouzifard, Carbohydr. Polym. 156, 340 (2017)
S. Berndt, F. Wesarg, C. Wiegand, D. Kralisch, F.A. Müller, Cellulose 20, 771 (2013)
A. Khalid, R. Khan, M. Ul-Islam, T. Khan, F. Wahid, Carbohydr. Polym. 164, 214 (2017)
W. Hu, S. Chen, J. Yang, Z. Li, H. Wang, Carbohydr. Polym. 101, 1043 (2014)
R. Alosmanov, K. Wolski, S. Zapotoczny, Cellulose 24, 285 (2017)
R.D. Pavaloiu, A. Stoica-Guzun, M. Stroescu, S.I. Jinga, T. Dobre, Int. J. Biol. Macromol. 68, 117 (2014)
X. Shi, Y. Zheng, G. Wang, Q. Lin, J. Fan, RSC Adv. 4, 47056 (2014)
O. Saibuatong, M. Phisalaphong, Carbohydr. Polym. 79, 455 (2010)
S.I. Jeong, S.E. Lee, H. Yang, Y.H. Jin, C.S. Park, Y.S. Park, Mol. Cell. Toxicol. 6, 370 (2010)
S. Salimi, R. Sotudeh-Gharebagh, R. Zarghami, S.Y. Chan, K.H. Yuen, ACS Sustain. Chem. Eng. 7, 15800 (2019)
G. Helenius, H. Backdahl, A. Bodin, U. Nannmark, P. Gatenholm, B. Risberg, J. Biomed. Mater. Res. A 76, 431 (2006)
D.A. Schumann, J. Wippermann, D.O. Klemm, Cellulose 16, 877 (2009)
I.F. Almeida, T. Pereira, N.H.C.S. Silva, Eur. J. Pharm. Biopharm. 86, 332 (2014)
I.R.S. Vieira, A.P.A.D. de Carvalho, C.A. Conte-Junior, Comp. Rev. Food Sci. Food Saf. 21, 3673–3716 (2022)
D. Kralisch, N. Hessler, D. Klemm, R. Erdmann, W. Schmidt, Biotechnol. Bioeng. 105, 740 (2010)
S.C. Wu, M.H. Li, J. Biosci. Bioeng. 120, 444 (2015)
M. Zeng, A. Laromaine, A. Roig, Cellulose 21, 4455 (2014)
Y. Qiu, L. Qiu, J. Cui, Q. Wei, Mater. Sci. Eng. C. Mater. Biol. Appl. 59, 303 (2016)
E. Kaplan, T. Ince, E. Yorulmaz, F. Yener, E. Harputlu, N.T. Laçin, J. Biomater. Tissue Eng. 4, 543 (2014)
S.N. Marghaki, M.Z. Jonoush, A. Rezaee, J. Clean. Prod. 277, 1 (2020)
A. Gupta, S.M. Briffa, S. Swingler, H. Gibson, V. Kannappan, G. Adamus, M. Kowalczuk, C. Martin, I. Radecka, Biomacromol 2020, 21 (1802)
F. Wahid, H. Bai, F. Wang, Y. Xie, Y. Zhang, L.Q. Chu, S.R. Jia, C. Zhong, Cellulose 27, 369 (2020)
S.S. Jaffar, S. Saallah, M. Misson, S. Siddiquee, J. Roslan, S. Saalah, W. Lenggoro, Membranes 12, 287 (2022)
S. Hamedi, S.A. Shojaosadati, V. Najafi, V. Alizadeh, Carbohydr. Polym. 229, 1 (2020)
A. Salama, A.K. Saleh, I.C. Maya, V. Guarino, J. Funct. Biomater. 14(60), 1–10 (2023)
J. Wei, W. Baoxiu, Z. Li, Z. Wu, M. Zhang, N. Sheng, Q. Liang, H. Wang, S. Chen, Carbohydr. Polym. 238, 116207 (2020)
V. Rivero-Buceta, M.R. Aguilar, A.M.H. Arriaga, F.G. Blanco, A. Rojas, M. Tortajada, R.A. Ramírez-Jiménez, B. Vázquez-Lasa, A. Prieto, Int. J. Biol. Macromol. 2020, 162 (1869)
S. Tang, K. Chi, H. Xu, Q. Yong, J. Yang, J.M. Catchmark, Carbohydr. Polym. 252, 117123 (2021)
C. Dou, Z. Li, J. Gong, Q. Li, C. Qiao, J. Zhang, Int. J. Biol. Macromol. 170, 354 (2021)
P. Weyell, U. Beekmann, C. Kupper, M. Dederichs, J. Thamm, D. Fischer, D. Kralis, Carbohydr. Polym. 207, 1 (2019)
S. Adepu, P. Kalyani, M. Khandelwal, Trans. Indian Natl. Acad. Eng. 6, 265 (2021)
H. Ullah, M. Badshah, A. Correia, F. Wahid, H. Santos, T. Khan, Curr. Pharm. Des. 24, 3692 (2019)
U. Beekmann, L. Schmolz, S. Lorkowski, O. Werz, J. Thamm, D. Fischer, D. Kralisch, Carbohydr. Polym. 236, 1 (2020)
K.M. Hosny, H.M. Alkhalidi, W.S. Alharbi, S. Md, A.M. Sindi, S.A. Ali, M. Kurakula, Polymers 14, 92 (2022)
J. Hua, C. Liu, P. Fai, N.B. Fei, Carbohydr. Polym. 259, 117737 (2021)
C. Busuioc, G.O. Isopencu, I.M. Deleanu, Appl. Sci. 13, 1015 (2023)
E. Trovatti, C.S. Freire, P.C. Pinto, Int. J. Pharm. 435, 83 (2012)
A. Müller, Z. Ni, N. Hessler, J. Pharm. Sci. 102, 579 (2013)
L.M.P. Sampaio, J. Padrão, J. Faria, J.P. Silva, C.J. Silva, F. Dourado, A. Zille, Carbohydr. Polym. 145, 1 (2016)
S.C. Wu, S.M. Wu, F.M. Su, J. Chem. Technol. Biotechnol. 92, 109 (2017)
E. Trovatti, N.H. Silva, I.F. Duarte, C.F. Rosado, I.F. Almedia, P. Costa, C.S.R. Freire, A.J.D. Silvestre, C.P. Neto, Biomacromol 12, 4162 (2011)
M. Rouabhia, J. Asselin, N. Tazi, Y. Messaddeq, D. Levinson, Z. Zhang, ACS. Appl. Mater. Interfaces 6, 1439 (2014)
E. Liyaskina, V. Revin, E. Paramonova, J. Phys. Conf. Ser. 784, 012034 (2017)
L. Stasiak-Rozanska, J. Ploska, Polymers 10, 438 (2018)
M. Das, O. Zandraa, C. Mudenur, N. Saha, P. Sáha, B. Mandal, V. Katiyar, ACS Appl. Bio Mater. 5, 3722–3733 (2022)
A.W. Indrianingsih, V.T. Rosyida, R. Suryani, S.M. Asari, S.I. Pratiwi, AIP Conf. Proc. 2606(1), 1–10 (2023)
G. Isopencu, I. Deleanu, C. Busuioc, O. Oprea, V.A. Surdu, M. Bacalum, R. Stoica, A.S. Guzun, Int. J. Mol. Sci. 24, 1719 (2023)
M. Perużyńska, A. Nowak, R. Birger, P.O. Rupniewska, M. Konopacki, R. Rakoczy, Ł Kucharski, K. Wenelska, A. Klimowicz, M. Droździk, M. Kurzawski, Bioeng. Biotechnol. 2, 1–9 (2023)
S. Bottan, F. Robotti, P. Jayathissa, A. Hegglin, N. Bahamonde, J.A. Heredia-Guerrero, I.S. Bayer, A. Scarpellini, H. Merker, N. Lindebnblatt, ACS Nano 9, 206 (2015)
C.N. Wu, S.C. Fuh, S.P. Lin, Y.Y. Lin, H.Y. Chen, J.M. Liu, K.C. Cheng, Biomacromol 19, 544 (2018)
M. Fürsatz, M. Skog, P. Sivlér, E. Palm, C. Aronsson, A. Skallberg, G. Greczynski, H. Khalaf, T. Bengtsson, D. Aili, Biomed. Mater. 13, 025014 (2018)
T. Maneerung, S. Tokura, R. Rujiravanit, Carbohydr. Polym. 72, 43 (2008)
G. Yang, J. Xie, Y. Deng, Y. Bian, F. Hong, Carbohydr. Polym. 87, 2482 (2012)
Y.H. Tsai, Y.N. Yang, Y.C. Ho, M.L. Tsai, F.L. Mi, Carbohydr. Polym. 180, 286 (2018)
M. de Lima-Fontes, A.B. Meneguin, A. Tercjak, J. Gutierrez, B.S.F. Cury, A.M. dos Santos, S.J.L. Ribeiro, H.S. Barud, Carbohydr. Polym. 179, 126 (2018)
R. Hobzova, J. Hrib, J. Sirc, E. Karpushkin, J. Michalek, O. Janouskova, P. Gatenholm, J. Nanomater. 2018, 1–11 (2018)
J. Wang, C. Gao, Y. Zhang, Y. Wan, Mater. Sci. Eng. 30, 214 (2010)
Y. Jia, X. Zhai, W. Fu, Y. Liu, F. Li, C. Zhong, Carbohydr. Polym. 151, 907 (2016)
X. Lv, J. Yang, C. Feng, Z. Li, S. Chen, M. Xie, Y. Xu, A.C.S. Biomater, Sci. Eng. 2, 19 (2016)
S. Gonçalves, I.P. Rodrigues, J. Padrão, J.P. Silva, V. Sencadas, S. Lanceros-Mendez, L.R. Rodrigues, Colloids Surf. 139, 1 (2016)
C.A. Santos, G.R. Santos, V.S. Soeiro, J.R. Santos, M.A. Rebelo, M.V. Chaud, Cellulose 25, 6681 (2018)
V.T. Nguyen, M.J. Gidley, G.A. Dykes, Food Microbiol. 25, 471 (2008)
H. Zhu, S. Jia, H. Yang, W. Tang, Y. Jia, Z. Tan, Food Sci. Biotechnol. 19, 1479 (2010)
M. Colic, S. Tomic, M. Bekic, Immunol. Lett. 222, 80 (2020)
S. Tomic, N. Ilic, V. Kokol, A. Gruden-Movsesijan, D. Mihajlovic, M. Bekic, L. Sofronic-Milosavljevic, M. Colic, D. Vucevic, Int. J. Nanomed. 13, 6941 (2018)
M. Andresen, P. Stenstad, T. Møretrø, S. Langsrud, K. Syverud, L.S. Johansson, P. Stenius, Biomacromol 8, 2149 (2007)
S. Sandhu, A. Arpa, X. Chen, R. Kumar, J. Jaworski, Recent Prog. Mater. 1, 30 (2019)
J. Singh, N.C.S. Tan, U.R. Mahadevswamy, N. Chanchareonsook, T.W.J. Steele, S. Lim, Carbohydr. Polym. 274, 118403 (2021)
M. Barjasteh, S.M. Dehnavi, S.A. Seyedkhani, S.Y. Rahnamaee, M. Golizadeh, Surf. Interfaces 36, 102631 (2023)
L. Bacakova, J. Pajorova, M. Bacakova, A. Skogberg, P. Kallio, K. Kolarova, V. Svorcik, Nanomaterials 9, 164 (2019)
J.D.P. de Amorim, C.J.G. da Silva-Junior, A.D.L.M. de Medeiros, H.A. do Nascimento, M. Sarubbo, T.P.M. de Medeiros, L.A. Sarubbo, Molecules 27, 5580 (2022)
V. Thakur, A. Guleria, S. Kumar, S. Sharma, K. Singh, Recent advances in nanocellulose processing, functionalization and applications: a review. Mater. Adv. 2, 1872–1895 (2021)
A. Meftahi, R. Khajavi, A. Rashidi, M. Sattari, M.E. Yazdanshenas, M. Torab, Cellulose 17, 199 (2010)
M. Scherner, S. Reutter, D. Klemm, A. Sterner-Kock, M. Guschlbauer, T. Richter, G. Langebartels, N. Madershahian, W.J. Wippermann, J. Surg. Res. 189, 340 (2014)
L. Bao, J. Tang, F. Honga, X. Lud, L. Chena, Carbohydr. Polym. 239, 1 (2020)
J.F. Godinho, F.V. Berti, D. Muller, C.R. Rambo, L.M. Porto, Cellulose 23, 545 (2016)
J.C. Astorga, K.T. Walker, N. Herrera, K.Y. Lee, T. Ellis, Nat. Commun. 12, 1 (2021)
L. Gu, T. Li, X. Song, X. Yang, S. Li, L. Chen, P. Liu, X. Gong, C. Chen, L. Sun, Regener. Biomater. 7, 195 (2020)
P.A. Rühs, K.G. Malollari, R. Binelli-Marco, C. Rowena, D.W.R. Balkenende, A.R. Studart, P.B. Messersmith, ACS Nano 14, 3885 (2020)
K. Wang, M. Qian, Y.-M. Zhang, G.-T. Han, C.-X. Qu, S.-D. Wang, Cellulose 27, 1845 (2020)
R. Patwa, O. Zandraa, Z. Capáková, N. Saha, P. Sáha, Polymers 12, 1 (2020)
T. Siripongpreda, B. Somchob, N. Rodthongkum, V.P. Hoven, Carbohydr. Polym. 256, 117506 (2021)
J. Hua, C. Liu, P.F. Ng, B. Fei, Carbohydr. Polym. 259, 117737 (2021)
D.C. Jusko, A. Zywicka, A. Junka, R. Drozd, P. Sobolewski, P. Migdal, U. Kowalska, M. Toporkiewicz, K. Fijalkowski, Carbohydr. Polym. 253, 117247 (2021)
T.J. Oliveira, T.C.M. Segato, G.P. Machado, D. Grotto, A.F. Jozala, Molecules 27, 8341 (2022)
J.D. Amorim, H.A. Nascimento, C.J.G. Silva Junior, A.D. Medeiros, I.D.L. Silva, A.F.S. Costa, L.A. Sarubbo, Polym. Eng. Sci. 62(2), 565–575 (2022)
N.H.C.S. Silva, I. Drumond, I.F. Almeida, P. Costa, C.F. Rosado, C.P. Neto, C.S.R. Freire, A.J.D. Silvestre, Cellulose 21, 665 (2014)
E.S. Morais, N.H.C.S. Silva, T.E. Sintra, S.A.O. Santos, B.M. Neves, I.F. Almeida, P.C. Costa, S.I. Correia, S.P.M. Ventura, A.J.D. Silvestre, Carbohydr. Polym. 206, 187 (2019)
G. Chantereau, M. Sharma, A. Abednejad, C. Vilela, E.M. Costa, M. Veiga, F. Antunes, M.M. Pintado, G. Sèbe, V. Coma, J. Mol. Liq. 302, 112547 (2020)
Y. Numata, H. Kono, M. Tsuji, K. Tajima, Carbohydr. Polym. 173, 67 (2017)
N. Chunshom, P. Chuysinuan, S. Techasakul, S. Ummartyotin, Sci. Adv. Mater. Devices 3, 296 (2018)
P. Perugini, M. Bleve, R. Redondi, F. Cortinovis, A. Colpani, J. Cosmet. Dermatol. 19, 725 (2020)
N.F. Vasconcelos, F.K. Andrade, L. de Araújo Pinto Vieira, R.S. Vieira, J.M. Vaz, P. Chevallier, D. Mantovani, M. de Fátima Borges, M. de Freitas Rosa, Cellulose 27, 3055 (2020)
S.H. Jun, S.G. Park, N.G. Kang, Polymers 11, 1044 (2019)
S. Silviana, A.N. Sa’adah, G.N.A. Milyawan, T. Nadya, AIP Conf. Proc. 2667(1), 1–9 (2023)
K. Wang, R.S. Hazra, Q. Ma, M.R.H. Khan, A.A. Hoque, L. Jiang, M. Quadir, Y. Zhang, S. Wang, G. Han, Cellulose 30, 3973–3993 (2023)
N.H. Fahim, M. Montazer, N. Hemmatinejad, T. Toliyat, M.M. Rad, Int. J. Biol. Macromol. 232, 123292 (2023)
D. Martins, B. Estevinho, F. Rocha, F. Dourado, M.A. Gama, Carbohydr. Polym. 230, 115657 (2020)
W.S. Chang, H.H. Chen, Food. Hydrocoll. 53, 75 (2016)
V.V. Revin, E.V. Liyaskina, M.V. Parchaykina, T.P. Kuzmenko, I.V. Kurgaeva, V.D. Revin, M.W. Ullah, Polymers 14, 4670 (2022)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no interest of conflict with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandey, A., Singh, M.K. & Singh, A. Bacterial cellulose: A smart biomaterial for biomedical applications. Journal of Materials Research 39, 2–18 (2024). https://doi.org/10.1557/s43578-023-01116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01116-4