Abstract
Diabetes mellitus (DM) is a chronic metabolic disease. Current therapies, including islet transplantation suffer instant blood mediated inflammatory reaction, nutrition and oxygen supply deficiency. Graphene oxide (GO) has shown to promote proliferation of different cells and alginate-based scaffolds are alternatives for beta-pancreatic cell functional improvement. We developed an alginate-GO based hydrogel that allows encapsulation and supporting beta-pancreatic cell survival. Physicochemical analysis revealed that a high GO concentration contributed to the morphological and chemical modification of the polymer matrix. Further analysis showed that alginate-GO hydrogel presented a more compact structure, less swelling, and lower degradation rate at high GO concentrations. Mechanical analysis revealed similar behaviour to that of the pancreas. Biocompatibility analysis demonstrated a relative increase in viability, proliferation, and cellular respiration due to GO content. 25 µg/mL alginate-GO hydrogel is a potential candidate for cell encapsulation and in vitro studies suggest a low cytotoxic effect in pancreatic cells, and enhanced functional behaviour, which may be favourable for diabetes treatment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a metabolic disease characterised by increased glucose levels and an imbalance that favours partial or complete destruction of beta-pancreatic cells, leading to insulin deficiency. The two most common types of DM include DM types I and II. Type I DM has an autoimmune origin, whereas type II DM has a multifactorial origin and is acquired according to poor lifestyle habits [1]. If not well-managed, it increases the risk of heart disease, stroke, kidney failure, blindness, and peripheral neuropathy [2, 3], which ultimately deteriorates the quality of life of patients and their families and generates high costs for health systems [4]. In 1980, the World Health Organization (WHO) estimated that approximately 108 million adults had diabetes, a number that has increased over the years. Since 2014, 422 million adults have been reported to have this disorder, and it is estimated that by 2030, approximately 578 million people will suffer from it, constituting a severe global public health problem [5].
Currently, several materials of natural or synthetic origin have been used for the elaboration of scaffolds to increase the replication of pre-existing beta cells or improve their function in vivo and in vitro through encapsulation methods [6, 7]. However, most of these scaffolds do not mimic the complexity of the extracellular matrix (ECM) composition and structure of pancreatic cells because they do not favour cell–cell or cell-ECM interactions and usually trigger an immune response to the materials used as grafts [6, 7]. Hence, maintaining these cells in culture remains a challenge because of their complex regulatory mechanisms, dependency on oxygen, difficulty in transporting nutrients, and low proliferation rate in vivo and in vitro.
Alginate encapsulation methods have yielded favourable results [8,9,10] because alginate is a structural polysaccharide found in seaweed that is non-toxic and exhibits a low immunogenic profile [11]. Studies in rodents and diabetic primates have successfully restored normoglycaemia and reduced insulin requirements without the need for immunosuppressive drugs [9, 10, 12]. For instance, Ozawa et al. [9] developed an alginate construct that allowed cell encapsulation and control of blood glucose concentration. However, some limitations were found, such as the foreign body reaction (FBR) which should be addressed in future studies. Bochenek et al.[12] also synthesised alginate spheres with decreased FBR to control blood glucose concentrations in an in vivo study. Nevertheless, there were some difficulties related to the maintenance and behaviour of the biomaterial. Hence, further development is needed for alginate-based biomaterials used in manufacturing capsules [13] because these hydrogels must be sufficiently resistant to the medium in which they are grafted and to the shearing forces generated in the transplant [6]. Alginate has some limitations, such as poor mechanical properties, concerning the requirements for its application. Therefore, it is necessary to reinforce it with other materials [14].
In this regard, graphene oxide (GO) can improve the physicochemical, mechanical, and biological properties of alginate-based materials because it is biocompatible and non-toxic, with excellent physical and mechanical properties [15]. GO is an oxygenated derivative of graphene with active oxygen-containing groups that can interact with membrane lipids and receptors and interfere with cell metabolism. This has resulted in numerous translational applications in biomaterials, tissue engineering, and biomedicine [14, 16,17,18]. Wang et al. [16] successfully developed a hydrogel with sodium alginate and embedded GO to facilitate the controlled release of an anticancer drug. In addition, Ciriza et al. [19] synthesised an alginate/GO microgel to encapsulate myoblasts to enhance cell viability, metabolic activity, and membrane integrity of embedded cells. However, the effects of alginate/GO hydrogels on beta-pancreatic cells have not been thoroughly investigated. Therefore, the objective of this study was to synthesise an alginate-based hydrogel with the addition of GO that allows encapsulation and supports beta-pancreatic cells with potential applications in DM treatment.
Results and discussion
Physicochemical characterisation of hydrogels
The produced GO had a low ash content of approximately 0.3%; therefore, the material was almost free of inorganic matter after complete ignition or oxidation of the organic matter. This constitutes a quality parameter used in elemental analysis to prepare samples for nutritional studies [20]. In our study, this indicated an appropriate cleaning step, suggesting that the obtained GO may be part of the hydrogel formulation without inorganic contamination, which may affect cell viability. GO had a high carbon (44.01%), oxygen content (52.97%), and low nitrogen (0.2%) and hydrogen (2.52%) contents, indicating the formation of oxygenated and hydroxylated functional groups on the surface of the graphene sheets. This resulted in a C/O ratio of 0.83, confirming a high degree of oxidation, accompanied by a large space between the layers owing to the space occupied by the functional groups [21].
After alginate and GO hydrogel synthesis (AL 1.87%, ALGO 10, ALGO 25, and ALGO 50), the morphological structures of the hydrogels were analysed using SEM, as shown in Fig. 1. The AL 1.87% hydrogel [Fig. 1(a)] showed a matrix with granular microspheres of uniform size dispersed on the surface, which is common in this type of hydrogel [22]. It has been reported that this morphology may be due to the interaction of alginate carboxyl groups (COO−) and amino groups (NH3+). Although this study used a hybrid chitosan/alginate hydrogel, the morphological characteristics of these beads were mostly due to the original alginate microstructure [22]. In addition, Brus et al. [23] reported similar observations and suggested that alginate beads cross-linked by bivalent ions (Ca2+) are essentially spherical particles that can be observed using SEM, as in our current work. In hydrogels with different concentrations of GO [Fig. 1(b), (c) and (d)], changes in the surface morphology were observed, revealing structures in the form of regular sheets in the matrix, which may be attributed to the GO embedded in the alginate hydrogel. This morphology is associated with the disruption of sp2 planar carbon sheets due to the interaction with sp3 hybridised carbon, also known as flakes [24, 25]. As these concentrations increased, greater roughness on the surface of the hydrogel and greater uniformity of the flakes were observed without any aggregation, indicating the successful incorporation of GO into the alginate matrix [26]. Wang et al. [16] and Ionita et al. [27] also showed the same alignment of GO sheets on the surface of the alginate hydrogel confirming that these GO structures tend to lie down in the alginate solution due to their two-dimensional structure and gravitational attraction. A uniform dispersion and degree of alignment of GO within the alginate matrix, along with good interfacial adhesion between GO and alginate, should lead to significant improvements in the thermal stability and mechanical properties of the hydrogels.
The alginate-GO (ALGO 10, ALGO 25, and ALGO 50) hydrogels were analysed by FT-IR, and the results are shown in Fig. 2. The FT-IR spectra of GO and alginate were similar to those reported in previous studies [27,28,29]. In the region between 3000 and 3600 cm−1, a wide absorption band was observed in all the samples studied, which was attributed to the vibration modes of the hydroxyl groups due to the absorbed water [30]. The following dominant functional groups are present in the GO spectrum: carbonyls (C=O) at ~ 1716.68 cm−1, carboxyls (COOH) at ~ 1619.88 cm−1, and epoxides at ~ 1037.79 cm−1 and ~ 969.11 cm−1 [27, 29]. These characteristic peaks in the FT-IR spectrum indicate that GO was successfully synthesised because of the presence of reactive GO groups [16, 27].
Pristine alginate, AL 1.87%, and hydrogels of alginate with GO showed similar emission signals, representing the same functional groups because the proportion of GO in the hydrogels was relatively small. Absorption bands between ~ 1600 cm−1 and ~ 1414.44 cm−1 were present [27, 29]. These are the characteristic peaks of the asymmetric and symmetric elongations of the carboxylate groups (COOH) of the alginate polymer [26, 27]. Additionally, these two peaks are most useful for investigating ion crosslinking by calcium in alginate hydrogels [26]. Moreover, the bands around ~ 1287.03 cm−1 and ~ 1025.39 cm−1 correspond to the stretching vibration of the C–O groups, while the bands at ~ 887 cm−1 and ~ 818.66 cm−1 are assigned to the stretching vibration of the C–O–C groups and attributed to its polysaccharide structure [27, 31]. According to the literature [32], the symmetric deformation band of the carboxyl group at 1395.98 cm−1 for pure alginate shifted towards 1414.14 cm−1 in alginate hydrogels when calcium ions were added to the solutions. This behaviour is evidence of the ionic crosslinking of the carboxylate groups, owing to formation of “egg-box” structures caused by the interaction of -COO− and the Ca2+ ions, which is in agreement with previous studies [16, 26, 27].Moreover, when this interaction occurred, the peak of the CH– asymmetric stretching vibration at 2920 cm−1 was weak. This may be explained by the “egg-box” structure, which limits CH– stretching and reduces changes in the dipole moment [26].
On the other hand, with the addition of GO, the peak at ~ 3312.65 cm−1 (O–H stretching vibration) in the 1.87% AL hydrogel broadened and shifted to smaller wavelengths ~ 3305.58 cm−1 for ALGO 10, ALGO 25, and ALGO 50 hydrogels, which may be attributed to the interaction of alginate and GO through strong intermolecular hydrogen bonds between alginate and GO [26]. The electrostatic attraction and hydrogen bonding between alginate and GO may improve the mechanical response [27].
The structural changes in GO synthesised from natural graphite were confirmed using Raman spectroscopy, as shown in Fig. 3(a). The D-band (at ~ 1300 cm−1) corresponds to sp3 hybridisation, whereas the characteristic G-band (at ~ 1500 cm−1) corresponds to sp2 hybridisation of the carbon atoms and the examination of ordered versus disordered crystal structures [21]. It has been reported that the D-band is attributed to local defects and disorders, while the G-band is assigned to the E2g phonon of carbon sp2 atoms of the graphite lattice [33]. The intensity ratio (ID/IG) of the D band/G band is often used to determine the degree of graphitisation; in our study, this ratio was 0.85 which is in agreement with previous reports [21, 33]. Hence, our findings demonstrate the degree of oxidation of the GO nanosheets (or sp3/sp2 carbon ratio) in the alginate/GO beads, indicating that the GO layers are turbostratic disordered, with partial lost of graphitization [17, 21].
The GO and hydrogels were studied at different temperatures using TGA, and the results of the thermal degradation of the AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 samples are shown in Fig. 3(b). According to the TGA of pristine GO, it degraded in two steps. In the first step, GO exhibited a low mass decrease of approximately 20% below 180 °C which is explained by evaporation of the absorbed water. In the second step, a major weight loss (70% of its mass) occurred, corresponding to the decomposition of labile oxygen-containing functionalities, as previously reported [21, 34]. Thus, we demonstrated the thermal stability of the synthesised GO, which is significant because between 34 °C and 41 °C, GO loses less than 1% of its mass. This makes it a very stable material, providing the hydrogel with the necessary stability to withstand the temperatures found in the human body.
The different hydrogels (AL 1.87%, ALGO 10, ALGO 25, and ALGO 50) subjected to the TGA test, as shown in Fig. 3(b), exhibited stable behaviour with constant mass loss from room temperature to 100 °C. The mass loss at this point was 90% owing to the amount of water loss in each sample. Subsequently, it was observed that the mass loss rate changed at 100 °C. This can be attributed to the burning of the carbon present in the alginate network; however, this did not occur in the embedded GO, resulting in a continuous loss of functional groups. This indicated that GO enhanced the thermal stability of the alginate matrix and retarded the pyrolysis of the composite beads, as previously suggested [26, 27]. Li et al. [26] and Ionita et al. [27] reported a similar TGA in an alginate/GO hydrogel, however, the mass loss of the composites significantly dropped at 150 °C. It has been suggested that alginate undergoes a thermal degradation process that constitutes a loss of volatile products through dehydration, thermal degradation of the polymer, and carbonisation [26]. In general, GO provided excellent thermal stability in the ALGO samples; however, since both AL 1.87% and ALGO hydrogels are mainly composed of alginate, their TGA curves are similar and stable [26].
To evaluate the crosslinking and interactions of the hybrid/composite gel (alginate/GO hydrogel), swelling tests were performed. The swelling capacities of the elaborated hydrogels are shown in parentheses: AL 1.87% (51.38%), ALGO 10 (49.95%), ALGO 25 (49.42%), and ALGO 50 (48.10%). The increase in the swelling ratio can be attributed to the migration of water into the network, which is guided by osmotic pressure [16]. According to our results, the swelling properties of alginate microcapsules are affected by the presence of GO because this additive significantly reduces the swelling effect. These results indicate that higher concentrations of GO in the alginate microcapsules improve their resistance to osmotic changes and subsequent rupture compared to the AL 1.87% microcapsules [19]. Additionally, a higher swelling capacity expands and deforms the hydrogels, weakening their mechanical toughness [27, 31]. It has been reported that the increase in the swelling capacity of alginate beads is generally associated with low Ca2+ content [26, 27]. However, this relationship was altered when GO was added, and both the calcium content and swelling degree gradually decreased. A possible explanation for this phenomenon is that not only aligned GO nanosheets and hydrogen bonds may restrict the mobility of alginate chains, but also strong interfacial interactions between alginate and GO, which may impair and weaken the diffusion of water molecules in the matrix [26, 27]. This would suggest that interacting groups are in proximity, reflecting in the ALGO hydrogel mechanical properties, because they would not be easily affected by the swelling forces, as discussed below [27, 31]. Therefore, GO addition to the hydrogel is beneficial because a stable swelling degree may maintain hydrogel shape and their mechanical properties, as seen in our hydrogels.
We also evaluated the degradation percentage of these hydrogels and found that the percentage of AL 1.87% was 34.75%, 33.63% for ALGO 10, 24.42% for ALGO 25, and 5.52% for ALGO 50. The degradation percentage decreased as the GO content of the hydrogel increased, indicating that the ALGO 50 hydrogel exhibited the lowest degradation rate. This behaviour may be attributed to the physical crosslinking of GO with alginate, which overcame the disintegration of the hydrogels. In addition, GO exhibited better thermal behaviour, as evidenced by the thermogravimetric analysis [Fig. 3(b)], positively influencing the hydrogel mass loss. In comparison, the AL 1.87% hydrogel exhibited the highest degradation rate of 34.75%, indicating that hydrogels with high GO content would take longer to degrade at 37 °C. The increase in the degradation percentage of AL 1.87% can be influenced by the expansion of the alginate polymer chains and the breakdown of the COO-Ca-OOC crosslinks (egg-box structures) by PO43− anions in the media [16, 35]. Thus, our results indicate that GO positively influences the degradation rate of the ALGO composites, suggesting that they would have more time to connect with the native tissue, making it suitable for grafting.
Mechanical characterisation of hydrogels
For rheological analysis, the storage modulus (G′) and loss or viscosity modulus (G″) were compared according to shear stress, as shown in Fig. 4. We show the storage (G′) and loss (G″) moduli of the AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 hydrogels versus the applied deformation [Fig. 4(a) and (b)]. The storage modulus was higher than the loss modulus (G′ > G″), with the elastic character prevailing over the viscous character of the material [36]. In contrast, all the composite samples presented a linear area where G' and G'' were practically independent of the deformation that occurred, indicating a linear viscoelasticity range (LVR), which is related to the stable and structured shape of the hydrogels [37]. This characteristic is important for biomedical applications of ALGO hydrogels because it determines their stability during processing and application handling [26].
The variation in the concentrations of GO used within each hydrogel influences the values of the storage modulus (G') and the values of the critical shear deformation (gc), which is determined as the value of maximum deformation in which the value of G´ remains constant. According to Fig. 4(a) and (b), for the AL 1.87%, ALGO 10, and ALGO 25 hydrogels, the values of gc are 0.025, 0.50 and 0.80%, respectively, while for the ALGO 50 hydrogel, the value of gc corresponds to 1.58%, which indicates that the latter has a higher LVR range, suggesting there is a positive effect on the structural stability of the hydrogel. This behaviour has also been reported for alginate/GO beads for protein adsorption [26] and alginate/GO composites with enhanced thermal and mechanical properties [27].
Figure 4(c) and (d) show the graphs of the oscillatory tests for AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 as a function of the angular frequency applied. In all cases, the viscoelastic behaviour of the hydrogels is shown, where the elastic modulus is predominant over the viscous modulus (G′ > G″), causing the material to behave as a solid structure. In addition, the storage (G′) and loss (G″) moduli of the hydrogel ALGO 50 were higher than those of the other hydrogels. In contrast, the ALGO 25 and ALGO 10 composites behaved in the same way as AL 1.87% under this low-strain (0.1%) condition, as shown in Fig. 4(a) and (b).
The relationship between stress (MPa) and strain was investigated using dynamic mechanical tests, and the results for each hydrogel are presented in Fig. 5. A nonlinear behaviour with upward concavity (strain hardening and water loss) is observed, which may be attributed to the densification of the structure or to the increase in the degree of crosslinking, generating a more rigid and brittle polymer network. In this case, when the load was applied to each hydrogel, the polymeric chains were modified and reoriented because the liquid began to emerge and the friction produced a hardening effect on the material [38].
The compression elastic modulus of each hydrogel was calculated using the slopes of the curves in the strain range (0.1–0.3), with values of 0.0046, 0.0046, 0.0055, and 0.0056 MPa for AL 1.87%, ALGO 10, ALGO 25, and ALGO 50, respectively. The elastic modulus values for the ALGO 50 and ALGO 25 hydrogels were the highest, which is due to the contribution of GO, improving the mechanical behaviour through a higher formation of reinforcing bridges between the polymeric matrix and carbonaceous additive [39]. However, this behaviour was not observed for the ALGO 10 hydrogel because the elastic behaviour was not different from that of AL 1.87%. This may be due to an increase in the water content reflected in the swelling ratio results discussed above, which acts as a plasticiser and causes a decrease in the rigidity of the composite, resulting in a lower elastic modulus [40]. In addition, this agrees with previous studies where the structure and hydrogen bonding of the alginate and GO composite presented a uniform stress distribution and reduced the presence of stress concentration centres, increasing the mechanical properties of the composite [26, 27, 39, 40].
According to Rice et al. [41], the Young’s modulus of a healthy pancreas is between 0.0014 and 0.025 MPa; therefore, we believe that our hydrogels exhibit an elastic behaviour similar to that of this organ. This suggests that the material attempts to mimic the elasticity of the pancreas, which could facilitate the maintenance of beta-pancreatic cells. Additionally, this could be useful for cell transplantation because implants with lower stiffness generally lead to reduced Foreign Body Response (FBR) [42].
Biocompatibility study
Quantitative and qualitative cell viability and proliferation in alginate/GO hydrogels were evaluated using MTT, PicoGreen, and live/dead assays as well as oxygen uptake and functional behaviour (Fig. 6). The MTT assay was performed to assess the effect on viability and cell density, considering the concentration of GO in the hydrogels, as shown in Fig. 6(a). The MTT results showed that the AL 1.87% hydrogel yielded an average viability of 93.74 ± 1.31% (p = 0.5242) compared to the Cell Culture Plastic (CCP), which indicates that it does not produce cytotoxicity in beta-pancreatic cells cultured in vitro. Similarly, for the ALGO 10 and ALGO 25 hydrogels, the viability percentages increased significantly compared with CCP, resulting in 132.91 ± 3.87% (p = 0.0045) and 142.27 ± 11.09% (p = 0.0009), respectively. However, ALGO 50 did not show statistical significance (p = 0.3761) with respect to CCP, with an average percentage of 112.62 ± 4.23%. These results are consistent with the findings of several authors, who showed that GO concentrations of 10 and 25 μg/mL had a significant positive effect on cell viability [19, 43, 44]. In contrast, Ciriza et al. [19] suggested that a concentration of 50 μg/mL GO would also significantly increase cell viability; however, the days evaluated (8 days) and the cell model (C2C12 myoblasts) used may explain the discrepancy because these variables may induce different cell behaviours over time. Notably, cell viability was not affected by the spherical, soft, and well-contoured shape of the developed hydrogels because this structure has been demonstrated to provide a high surface-to-volume ratio, which is an ideal environment for supporting a large number of cells [42, 45].
Effects of alginate/GO hydrogels on BRIN BD-11 cells. (a) MTT assay results showing the percentage of viable cells after a culture period of 24 h in AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 media. (b) PicoGreen assay results showing the total DNA content of cells expressed as the proliferation percentage of the cultured samples. A standard curve of known dsDNA (ng/mL) was used to calculate the DNA content of the samples. (c) Live/dead staining of beta-pancreatic cells in the control (CCP), AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 samples. (Green = FDA (live); red = PI (dead)); scale bar = 200 μm. (d) Effect on oxygen flow in all experimental groups expressed as O2 flow pmol/(s × 106 cells). N ≥ 3, *p < 0.05, **p < 0.01, and ***p < 0.001. e) Insulin secretion in response to low and high glucose concentrations from alginate/GO hydrogels expressed as insulin secreted (μIU/mL × μg DNA). N ≥ 3, (a) vs. CCP, (b) vs. AL 1.87%., p < 0.05. Data points represent mean ± SEM.
To assess whether the concentration of GO in the hydrogels increased cell proliferation, a PicoGreen assay was performed. Figure 6(b) shows that ALGO 10 and ALGO 25 promoted significant cell proliferation with an average percentage of 123.33 ± 5.98% (p = 0.0417) and 132.99 ± 6.55% (p = 0.0033), respectively. However, the hydrogel ALGO 50 did not influence cell proliferation (p = 0.7933), as it resulted in an average value of 92.38 ± 5.86% and maintained fluorescence levels, as well as AL 1.87%, with a mean result of 92.43 ± 1.49% (p = 0.7966).
These observations agree with a previous finding, which demonstrated that GO promotes cell proliferation at low concentrations in a different cell line [46], as seen in ALGO 10 and ALGO 25 in the current study. The mechanism of GO activity might explain the current in vitro proliferation findings because GO adsorbs extracellular matrix (ECM) proteins through hydrophobic interactions, electrostatic forces, and hydrogen bonding, which promotes cell adhesion to GO and, hence, cell proliferation [25, 47, 48]. It should be noted that GO concentrations lower than 50 μg/mL are considered negligible, and this concentration is suggested to be the cytotoxic threshold in mammalian cell lines. GO cytotoxicity is influenced by dose-dependent heavy metal impurities, which are important factors that might explain the cell viability and proliferation results in ALGO 50 [43, 49]. Additionally, Espona-Noguera et al. [40] suggested that pancreatic cell lines encapsulated in stiff alginate hydrogels cannot proliferate because this causes greater mechanical cell constriction and nutrient diffusion problems. This agrees with our findings because the ALGO 50 hydrogel possesses the highest elastic modulus and acts as a solid structure, as discussed in the previous section.
A live/dead viability assay was performed to verify the integrity of BRIN-BD11 cells after encapsulation in the four prepared hydrogels (AL 1.87%, ALGO 10, ALGO 25, and ALGO 50), as shown in Fig. 6(c). The cells were viable after 24 h of culture, corroborating the results of MTT assay. The images corresponding to the ALGO 10, ALGO 25, and ALGO 50 hydrogels showed more visible viable cells than those in the AL 1.87% hydrogel, as previously reported [19, 43, 44]. Therefore, the results shown in Fig. 6(a), (b) and (c) suggest that the alginate/GO system is viable for the maintenance of beta-pancreatic cells because there was no impairment in the viability, proliferation, or plasma membrane integrity of these cells. Our data demonstrate the usefulness of ALGO 10 and ALGO 25, as they favour the viability and proliferation of beta-pancreatic cells and may be used as potential platforms for diabetes treatment.
Next, we determined the effect of alginate/GO encapsulation on the oxygen consumption flow of beta-pancreatic cells [Fig. 6(d)]. This is an indicator of mitochondrial respiration in different respiratory states, including: resting, leak, and uncoupled [50, 51]. The resting respiratory capacity in ALGO 10 (p = 0.2158) and ALGO 25 (p = 0.0779) was not significantly affected by the cell-loaded platform compared to CCP. Nevertheless, AL 1.87% (p = 0.0467) and ALGO 50 (p = 0.0218) showed significant impairment in basal respiration compared to CCP. Moreover, it can be observed that leak respiration showed no significant differences in any condition (AL 1.87%, p = 0.1654; ALGO 10, p = 0.9031; ALGO 25, p = 0.2283; ALGO 50, p = 0.5068), whilst the uncoupled state (maximal respiratory capacity) had a significant decreased in ALGO 50 (p = 0.0236) that was no significant in AL 1.87% (p = 0.0967), ALGO 10 (p = 0.1043), and ALGO 25 (p = 0.0832) with respect to the CCP.
Our results suggest that ALGO 10 and ALGO 25 did not affect energy metabolism related to mitochondrial respiration. However, AL 1.87% showed a drastic reduction in basal respiratory capacity, which is in agreement with previous reports [50, 50,51,52]. These findings suggest that encapsulation in alginate alone is not beneficial for pancreatic cells, and functionalisation of the biomaterial is needed to improve the metabolic responsiveness of the cells. In this context, the use of GO has led to tremendous advances in biomedical applications, including tissue engineering and regenerative medicine [18, 53]. Nonetheless, the effect of GO is highly dependent on the experimental conditions, including the time of exposure, dose, type of cells, and analytical methods used to establish the effect on cell behaviour [54]. This is important because the results of the ALGO 50 condition suggest impairment of mitochondrial respiratory capacity, which might include the Oxidative Phosphorylation System (OXPHOS) [55]. Previous proteomic analyses have revealed that GO concentrations greater than 40 μg/mL not only selectively downregulated the expression of OXPHOS-related genes but also inhibited OXPHOS and oxygen uptake [55, 56]. Additionally, in these studies, a positive correlation was found between GO concentration and its effect on normal and cancer cell cytotoxicity, which is reflected in the impairment of energy metabolism. Our in vitro cell model, BRIN-BD11, is a hybrid cell line derived from a rat insulinoma that may partially explain the respiratory capacity inhibition in the ALGO 50 experimental condition [57]. However, some studies have reported that the activity of mitochondrial complexes is not drastically inhibited at low GO concentrations, including 20 μg/mL, which may be directly observed in the mitochondrial respiratory capacity [55, 58]. In the current study, we suggest that ALGO 10 and ALGO 25 are optimal conditions that do not impair oxygen uptake capacity in any mitochondrial respiratory state. One possible explanation is that the degree of functionalisation of GO provides sufficient oxygen content to overcome the toxic effects of graphene [59]. However, the dose-dependent cytotoxicity of GO explained above should also be considered [55, 56].
To evaluate the functional behaviour of beta-pancreatic cells, we performed a glucose-stimulated insulin secretion (GSIS) assay [Fig. 6(e)]. ALGO 50 condition was not included because the cumulative results did not demonstrate that this was an optimal condition for proliferation and oxygen flow, as previously discussed. Embedded beta-pancreatic cells in alginate/GO hydrogels were exposed to 2.8 mM and 28 mM glucose solutions. At the low glucose concentration, CCP and AL 1.87% had a similar secretion behaviour (p = 0.3882). However, at this low concentration, ALGO 10 and ALGO 25 produced significantly higher insulin secretion than CCP, averaging 0.7060 ± 0.0088 (p = 0.0040) and 0.6874 ± 0.0167 (p = 0.0102) μIU/mL × μg DNA, respectively. At high glucose concentration, we observed a statistically significant increase in insulin secretion per μg DNA only for ALGO 25, averaging 0.8696 ± 0,0297 (p = 0.0163) compared to CCP. Additionally, we also found that ALGO 25 had a significant (p = 0.0095) insulin release when stimulated at a high glucose concentration, compared to AL 1.87%. These results suggest that 25 μg/mL GO enhances insulin secretion in encapsulated beta-pancreatic cells. A previous study [60] suggested that the addition of CaCl2 in the crosslinking process of functionalised alginate hydrogels triggers specific signalling processes in beta-pancreatic cells, enhancing insulin secretory responses. This interaction leads to an elevation of intracellular Ca2+; hence, the activation of different mechanisms leads to the activation of the insulin secretory pathway [60]. This would partially explain our functional results because the hydrogels underwent a crosslinking process with 100 mM CaCl2; however, further studies should be performed. In comparison, functionalised alginate hydrogels with extracellular matrix components and peptides have been reported to enhance functional behaviour and amplify insulin secretion when these molecules are added [8, 51]. In this regard, we believe that GO interaction with ECM components may influence cell behaviour since it is known that cell–matrix and biochemical signalling interactions are needed for insulin secretion [61]. This would explain why ALGO 25 induced a higher baseline insulin secretion (low glucose) as well as in the stimulatory glucose concentration (high glucose). Furthermore, another possible explanation is that the insulin secretory pathway is influenced in response to a combination of the stiffness, stress relaxation, and adhesion ligands of the corresponding cellular microenvironments that can be found in a 3D viscoelastic material such as ALGO 25 [61].
Based on the results presented, our study revealed a harmonious interaction between alginate and GO in a tailored biomaterial. We recommend ALGO 25 as the best condition because it shows an acceptable swelling rate and low degradation percentage, coupled with the presence of alginate, and GO chemical groups. Similarly, this biomaterial, with its viscoelastic profile, attempts to mimic the elasticity of the pancreas along with satisfactory thermal stability, which could be useful for implantation and graft survival. Moreover, higher survival capability and proliferation capacity, along with better energy metabolism performance and functional behaviour, were observed in ALGO 25. This suggests that alginate with a GO concentration of 25 μg/mL possesses adequate physicochemical, biomechanical, and biocompatibility properties to encapsulate beta-pancreatic cells with enhanced functionality for potential application in DM treatment.
Conclusion
In this study, alginate-graphene oxide hydrogels were successfully developed and live beta-pancreatic cells were encapsulated through ionic gelation. Physicochemical characterisation demonstrated that GO affected the intermolecular interactions and mechanical behaviour of the alginate hydrogel, which may be favourable for translational applications. Morphological analysis revealed that GO was incorporated into the alginate matrix, with regular sheets visible throughout the hydrogel surface. We demonstrated that ALGO 25 presented the best viability, proliferation percentage, and respiratory capacity with encapsulated beta-pancreatic cells, as well as the best mechanical and thermal properties exhibited by the composite. In addition, this condition enhanced the functional behaviour of encapsulated beta-pancreatic cells. To the best of our knowledge, this is the first study to evaluate alginate-GO hydrogels as a beta-pancreatic cell-laden platform with promising results. In this regard, the study outcomes may establish an ideal candidate for preclinical evaluation of diabetes treatment and functionalisation of the construct in future studies.
Materials and methods
Materials
Graphite powder, HCl 30%, H2SO4 98% and HNO3 65% were purchased from Merck (Darmstadt, Germany), ultrapure water was obtained from a water purification system (Elga PURELAB DV 25, Veolia Water Systems, Dublin, Ireland), and calcein AM, ethidium homodimer-1, and Quant-iT™ PicoGreen were purchased from Invitrogen (Thermo Scientific, Waltham, MA, USA). All other chemicals and materials were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Graphene oxide synthesis
Graphene oxide (GO) was synthesised using the modified Hummers method [62]. The typical procedure for synthesis was as follows: 2 g of graphite, 120 mL of H2SO4, and 40 mL HNO3 were mixed. The suspension was placed in an ice bath and stirred for 20 min. Subsequently, 10 g of KMnO4 was added in small portions. Once completed, the ice bath was removed, stirring was continued for 2 h, and 400 mL of distilled water was added to quench the reaction. Then 8.6 mL of H2O2 was added. When the temperature of the suspension decreased, 100 mL 10% HCl was added to the mixture. After continuous cycles of centrifugation and washing with distilled water, the dissolved GO species were removed until the residue reached almost neutral pH. Finally, GO was obtained by drying the gel for 12 h at 80 °C in a vacuum oven, as previously described [62]. The GO suspension was prepared at 1 mg/mL, followed by sonication for the hydrogel preparation.
Manufacture of alginate and GO hydrogel
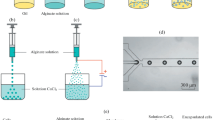
To prepare the alginate and GO hydrogels, an alginate 1.87% (w/v, AL 1.87%) solution was obtained, and different concentrations of GO [10 μg/mL (ALGO 10), 25 μg/mL (ALGO 25), 50 μg/mL (ALGO 50)] were prepared in alginate solution. The samples were extruded, and the pH was adjusted to 7.0–7.4. Finally, to favour ionic gelation of the resulting alginate and GO beads (AL 1.87%, ALGO 10, ALGO 25, and ALGO 50), the beads were placed in contact with a CaCl2 solution (100 mM) and stirred at 230 rpm for 15 min.
Physicochemical characterisation
To evaluate the purity and chemical composition of GO, elemental analysis was performed on a Sundy SDCHN435 Carbon Hydrogen and Nitrogen Analyzer (Hunan Sundy Science and Technology Co., China), with an analysis time per sample of ≤ 5 min and helium consumption of 0.7 L/min.
Subsequently, the ash content was analysed to identify impurities present in the GO by heating the sample to a temperature 450–500 °C for one hour. The mixture was maintained for another 1 h at a temperature of 700–750 °C. The sample was extracted, cooled in a desiccator, and weighted [63].
To obtain information on the morphological, structural, and chemical characteristics of the alginate hydrogel with GO, the scanning electron microscopy (SEM) technique was performed with the QUANTA FEG 650 electron microscope (FEI Company, Oregon, USA). The samples were dehydrated and coated with gold. Imaging was performed in ultra-vacuum at a voltage of 25 kV using secondary electrons (SE) and backscattered electrons (BSE). For the chemical analysis, X-ray energy dispersive spectroscopy (EDS) was used with an acceleration voltage of 25 kV.
Fourier-transform infrared spectroscopy (FT-IR) was used to study and verify the incorporation of monomers into the structure of the hydrogels and to observe the presence of the characteristic functional groups of each monomer. A CARY 630 FTIR spectrophotometer (Agilent, California, USA) with attenuated total reflectance (ATR) was used, and the spectra of the samples were recorded at room temperature in transmittance mode; eight scans were performed at 4000–600 cm−1. The spectra of GO, pure alginate, and AL 1.87% hydrogels were used as the controls.
Raman analysis was performed to determine the degree of graphitisation of the GO. A LabRAM HR Evolution Raman-HORIBA spectrophotometer (HORIBA Ltd., Kyoto, Japan) was used.
To evaluate the mass of the GO composites within the hydrogels at different temperatures, thermogravimetric analysis (TGA) was carried out using a TGA 5500 thermogravimetric analyser (TA Instruments, Inc.) in the temperature range of 0–400 °C at a heating rate of 10 °C min−1 for AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 under a constant nitrogen atmosphere, and a temperature range of 0–600 °C at a heating rate of 10 °C/min for GO alone with oxygen.
The in vitro degradation property was characterised by evaluating the weight loss ratio [64]. The weight loss ratio was calculated from the average value of the weight change for nine samples per condition. The mathematical formula used to calculate the weight loss ratio of the samples is given by Eq. (1):
where the initial weight (Wi) is the weight of the samples before the introduction of hydrogels into the culture medium and the final weight (Wf) is the weight of the samples after 24 h of incubation at 37 °C and complete drying.
Mechanical characterisation
Dynamic mechanical analysis (DMA) was performed. Compression tests were performed on the AL 1.87%, ALGO 10, ALGO 25, and ALGO 50 hydrogels to determine their maximum deformation and Young's modulus. This was carried out in the DMA equipment Q800 (TA Instruments, New Castle, USA), using a pre-load of 0.001 N and a ramp of 0.5 N/min at a temperature of 37 °C. The Young's modulus for each hydrogel tested was obtained from the slope of the linear region of the stress–strain curves.
Rheological studies were carried out using an MCR 302 ANTON PAAR rheometer (Anton-Paar, Graz, Austria) with 20 mm diameter parallel plates and a Peltier system for temperature control. To determine the storage modulus (G′) and the loss modulus (G″), amplitude sweeps were performed, with a deformation: 0.01–100%, and an angular frequency of 10 rad/s and frequency sweeps with a deformation of 0.1% for all samples.
Additionally, the swelling capacity of the hydrogels was evaluated to determine the mechanical response to the amount of water absorbed in their structure. The hydrogels were initially weighed, and the data were recorded as the initial weight (Wi). Nine hydrogels were incubated for 24 h at 37 °C in culture medium. The medium and each hydrogel were dried using filter paper to remove excess. The final weight was recorded as Wf and the swelling percentage was calculated using Eq. (2) [65]:
Beta-pancreatic cell encapsulation
BRIN-BD11 cells (10,033,003, ECACC) were cultured at a density of 1 × 105 cells/mL in RPMI-1640 medium supplemented with 10% (v/v) foetal bovine serum (FBS) and 1% (v/v) antibiotic–antimycotic solution at 37 °C in an atmosphere of 5% CO2. After confluence and to carry out the assays, cells were harvested with 0.25% (w/v) trypsin–EDTA solution and suspended in the solutions AL 1.87%, ALGO 10, ALGO 25, and ALGO 50. Ionic gelation through extrusion is the mechanism used for cell encapsulation [66]. The final capsules had a diameter of 4–6 mm. BRIN-BD11 non-encapsulated cells were used as controls (Cell Culture Plastic-CCP).
Cell viability
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and live/dead assays were performed [67]. Briefly, cells were encapsulated in each hydrogel and incubated for 24 h. After this, samples were integrated with MTT reagent at a concentration of 0.5 g/L and RPMI-1640 culture medium for 5 h. The supernatant was removed, DMSO was added, and the mixture was gently shaken for 30 min to allow the dissolution of the formazan crystals. The absorbance was measured at 570 nm using a microplate reader Synergy H1 (Agilent Technologies, Santa Clara, CA, USA). Live/dead staining was performed to confirm cell viability. ALGO samples were cultured for 24 h and stained for 45 min at room temperature with 5 μg/mL calcein AM and 5 μg/mL ethidium homodimer-1 (Molecular Probes, Eugene, Oregon, USA). Stained cells were visualised by fluorescence imaging using an Olympus microscope (Olympus America Inc. NY, USA).
Proliferation assay
The amount of DNA in the encapsulated cells on the ALGO samples was determined using the Quant-iT™ PicoGreen® dsDNA Kit [68]. Briefly, samples were collected after 48 h of incubation, rinsed three times with PBS and submerged in lysis buffer containing 10 mM Tris (pH 8), 1 mM EDTA, and 0.2% (v/v) Triton X-100. To release DNA, the samples were vortexed for 30 min and kept on ice throughout the entire process. The samples were thawed on ice and homogenised for 10–15 min. The sample was mixed with 100 μL of DNA-binding fluorescent dye solution. Fluorescence intensity was measured at an excitation wavelength of 480 nm and an emission wavelength of 520 nm using a Synergy H1 microplate reader (Agilent Technologies, Santa Clara, USA). Lambda DNA was used as the standard curve to calculate DNA content.
Respiratory capacity
To evaluate the mitochondrial respiratory capacity of encapsulated beta-pancreatic cells, oxygen uptake was measured via High-Resolution Respirometry with an Oxygraph-2 k (Oroboros Instruments, Innsbruck, Austria). In brief, two chambers of 2 mL were filled with culture medium at 37 °C under gentle agitation (750 rpm). Encapsulated cells (6 × 105 cells/mL) for each condition and the control were cultured for 24 h in RPMI-1640 medium. Each experiment was repeated at least three times. Oxygen consumption was assessed in different respiratory states as previously described [69]. Briefly, respiratory states were defined as follows: “resting” when oxygen is consumed in the absence of inhibitors or uncouplers, and “leak” when respiration occurred in the presence of 2.5 μM oligomycin. The “uncoupled” state refers to the maximal oxygen consumption in the presence of 1 μM FCCP (two titrations of 0.5 μM). The oxygen flow in these states was corrected by subtracting the non-mitochondrial respiration obtained after the addition of 2.5 μM rotenone and 2.5 μM antimycin. The oxygen solubility factor was 0.89 for RPMI-1640 and the local barometric pressure was 91 kPa. The results were expressed as the oxygen flow per cell [pmol/ (seg*1 × 106 cells)].
Functional behaviour
To assess the effect of functionalised collagen microgels on beta-pancreatic cell functionality, the static glucose-stimulated insulin secretion (GSIS) assay was performed [70]. Briefly, alginate hydrogels were incubated with Krebs–Ringer Bicarbonate (KRBH) buffer (125 mM NaCl, 3 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM NaH2PO4, 22 mM NaHCO3, 10 mM HEPES, and 0.1% BSA (Sigma-Aldrich, United States)), to a basal level of glucose (1.1 mM) for 30 min. This buffer was removed, and constructs were treated with low glucose (LG)-KRBH (2 mM) for 1 h, and high glucose (HG)-KRBH for 1 h. Samples from the LG-KRBH and HG-KRBH solutions were retained and collected. Insulin levels were detected using a Rat Insulin ELISA kit (Invitrogen, Thermo Scientific, United States). Finally, insulin secretion was normalised to DNA content (μg/mL) using the Quant-iT PicoGreen assay.
Statistical analysis
Statistical analysis was performed using the SPSS/Windows version 15.0 software (SPSS Inc. Chicago, IL, USA) and GraphPad Prism, Version 7 (GraphPad Software Inc., USA). All experimental results are presented as the mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to compare multiple cohorts, followed by post hoc Tukey’s test. In all cases, p < 0.05 was used to denote statistical significance, where p-values were p ≤ 0.05 (*), ≤ 0.001(**), and < 0.001(***).
Data availability
Data supporting the findings of this study are available to the corresponding author upon request. The data are not publicly available due to privacy or ethical restrictions.
References
A. Salsali, M. Nathan, A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am J. Ther. 13(4), 349 (2006)
F.M. Ashcroft, P. Rorsman, Diabetes mellitus and the β cell: the last ten years. Cell 148(6), 1160 (2012)
International Diabetes Federation, IDF diabetes atlas, 5th edn. (International Diabetes Federation, Brussels, 2011)
J.A. Seiglie, D. Nambiar, D. Beran, J.J. Miranda, To tackle diabetes, science and health systems must take into account social context. Nat. Med. 27(2), 193 (2021)
World Health Organization, Preventing disease through healthy environments: A global assessment of the environmental burden of disease. Toxicol. Lett. 259, S1 (2016)
D.R. Arifin, M. Kulkarni, D. Kadayakkara, J.W.M. Bulte, Fluorocapsules allow in vivo monitoring of the mechanical stability of encapsulated islet cell transplants. Biomaterials 221, 119410 (2019)
A. Espona-Noguera, J. Ciriza, A. Cañibano-Hernández, G. Orive, R.M. Hernández, L. Saenz del Burgo, J. Pedraz, Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics 11(11), 597 (2019)
J. Crisóstomo, A.M. Pereira, S.J. Bidarra, A.C. Gonçalves, P.L. Granja, J.F. Coelho, C.C. Barrias, R. Seiça, ECM-enriched alginate hydrogels for bioartificial pancreas: an ideal niche to improve insulin secretion and diabetic glucose profile. J. Appl. Biomater. Funct. Mater. 17(4), 228080001984892 (2019)
F. Ozawa, S. Nagata, H. Oda, S.G. Yabe, H. Okochi, S. Takeuchi, Lotus-root-shaped cell-encapsulated construct as a retrieval graft for long-term transplantation of human iPSC-derived β-cells. iScience 24(4), 102309 (2021)
B.L. Strand, A.E. Coron, G. Skjak-Braek, Current and future perspectives on alginate encapsulated pancreatic Islet. Stem Cells Transl. Med. 6(4), 1053 (2017)
O. Smidsrod, G. Skjakbrk, Alginate as immobilization matrix for cells. Trends Biotechnol. 8, 71 (1990)
M.A. Bochenek, O. Veiseh, A.J. Vegas, J.J. McGarrigle, M. Qi, E. Marchese, M. Omami, J.C. Doloff, J. Mendoza-Elias, M. Nourmohammadzadeh, A. Khan, C.-C. Yeh, Y. Xing, D. Isa, S. Ghani, J. Li, C. Landry, A.R. Bader, K. Olejnik, M. Chen, J. Hollister-Lock, Y. Wang, D.L. Greiner, G.C. Weir, B.L. Strand, A.M.A. Rokstad, I. Lacik, R. Langer, D.G. Anderson, J. Oberholzer, Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2(11), 810 (2018)
G. Orive, D. Emerich, A. Khademhosseini, S. Matsumoto, R.M. Hernández, J.L. Pedraz, T. Desai, R. Calafiore, P. de Vos, Engineering a clinically translatable bioartificial pancreas to treat type I diabetes. Trends Biotechnol. 36(4), 445 (2018)
Á. Serrano-Aroca, L. Iskandar, S. Deb, Green synthetic routes to alginate-graphene oxide composite hydrogels with enhanced physical properties for bioengineering applications. Eur. Polym. J. 103, 198 (2018)
J. Zhao, L. Liu, F. Li, Graphene Oxide: Physics and Applications (Springer, Berlin, 2015)
J. Wang, C. Liu, Y. Shuai, X. Cui, L. Nie, Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf. B Biointerfaces 113, 223 (2014)
C. Zhang, Y. Zhang, H. Shao, X. Hu, Hybrid silk fibers dry-spun from regenerated silk fibroin/graphene oxide aqueous solutions. ACS Appl. Mater. Interfaces 8(5), 3349 (2016)
X. Ding, H. Liu, Y. Fan, Graphene-based materials in regenerative medicine. Adv. Healthc. Mater. 4(10), 1451 (2015)
J. Ciriza, L. Saenz del Burgo, M. Virumbrales-Muñoz, I. Ochoa, L.J. Fernandez, G. Orive, R.M. Hernandez, J.L. Pedraz, Graphene oxide increases the viability of C2C12 myoblasts microencapsulated in alginate. Int. J. Pharm. 493(1–2), 260 (2015)
K. Liu, Effects of sample size, dry ashing temperature and duration on determination of ash content in algae and other biomass. Algal Res. 40, 101486 (2019)
E.M. Aliyev, M.M. Khan, A.M. Nabiyev, R.M. Alosmanov, I.A. Bunyad-zadeh, S. Shishatskiy, V. Filiz, Covalently modified graphene oxide and polymer of intrinsic microporosity (PIM-1) in mixed matrix thin-film composite membranes. Nanoscale Res. Lett. 13(1), 359 (2018)
E. Ablouh, Z. Hanani, N. Eladlani, M. Rhazi, M. Taourirte, Chitosan microspheres/sodium alginate hybrid beads: an efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Environ. Res. 29(1), 5 (2019)
J. Brus, M. Urbanova, J. Czernek, M. Pavelkova, K. Kubova, J. Vyslouzil, S. Abbrent, R. Konefal, J. Horský, D. Vetchy, J. Vysloužil, P. Kulich, Structure and dynamics of alginate gels cross-linked by polyvalent ions probed via solid State NMR spectroscopy. Biomacromol 18(8), 2478 (2017)
X. Colom, J. Cañavate, M.J. Lis, G. Sanjuan, Análisis estructural de Óxidos de Grafeno (GO) y Óxidos de Grafeno reducidos (rGO). Afinidad 77(591), 167–174 (2020)
J. Park, B. Kim, J. Han, J. Oh, S. Park, S. Ryu, S. Jung, J.-Y. Shin, B.S. Lee, B.H. Hong, D. Choi, B.-S. Kim, Graphene oxide flakes as a cellular adhesive: prevention of reactive oxygen species mediated death of implanted cells for cardiac repair. ACS Nano 9(5), 4987 (2015)
J. Li, J. Ma, S. Chen, Y. Huang, J. He, Adsorption of lysozyme by alginate/graphene oxide composite beads with enhanced stability and mechanical property. Mater. Sci. Eng. C 89, 25 (2018)
M. Ionita, M.A. Pandele, H. Iovu, Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 94(1), 339 (2013)
L. Pan, Z. Wang, Q. Yang, R. Huang, Efficient removal of lead, copper and cadmium ions from water by a porous calcium alginate/graphene oxide composite aerogel. Nanomaterials 8(11), 957 (2018)
L. Liu, C. Li, C. Bao, Q. Jia, P. Xiao, X. Liu, Q. Zhang, Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 93, 350 (2012)
X. Yang, X. Zhang, Z. Liu, Y. Ma, Y. Huang, Y. Chen, High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J. Phys. Chem. C 112(45), 17554 (2008)
B. Smitha, S. Sridhar, A.A. Khan, Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 41(8), 1859 (2005)
C. Sartori, D.S. Finch, B. Ralph, K. Gilding, Determination of the cation content of alginate thin films by FTi.r. spectroscopy. Polymer 38(1), 43 (1997)
K.N. Kudin, B. Ozbas, H.C. Schniepp, R.K. Prudhomme, I.A. Aksay, R. Car, Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8(1), 36 (2008)
A.M. Shanmugharaj, J.H. Yoon, W.J. Yang, S.H. Ryu, Synthesis, characterization, and surface wettability properties of amine functionalized graphene oxide films with varying amine chain lengths. J. Colloid Interface Sci. 401, 148 (2013)
J. Zhang, Q. Wang, A. Wang, In situ generation of sodium alginate/hydroxyapatite nanocomposite beads as drug-controlled release matrices. Acta Biomater. 6(2), 445 (2010)
T.G. Mezger, The rheology handbook: For users of rotational and oscillatory rheometers, 2nd edn. (Vincentz, Hanover, 2006)
R. Wong, M. Ashton, K. Dodou, Effect of crosslinking agent concentration on the properties of unmedicated hydrogels. Pharmaceutics 7(3), 305 (2015)
R. Ma, D. Xiong, F. Miao, J. Zhang, Y. Peng, Novel PVP/PVA hydrogels for articular cartilage replacement. Mater. Sci. Eng. C 29(6), 1979 (2009)
Y. Hu, W. Han, G. Huang, W. Zhou, Z. Yang, C. Wang, Highly stretchable, mechanically strong, tough, and self-recoverable nanocomposite hydrogels by introducing strong ionic coordination interactions. Macromol. Chem. Phys. 217(24), 2717 (2016)
A. Espona-Noguera, J. Ciriza, A. Cañibano-Hernández, L. Fernandez, I. Ochoa, L. Saenz del Burgo, J.L. Pedraz, Tunable injectable alginate-based hydrogel for cell therapy in type 1 diabetes mellitus. Int. J. Biol. Macromol. 107, 1261 (2018)
A.J. Rice, E. Cortes, D. Lachowski, B.C.H. Cheung, S.A. Karim, J.P. Morton, A. del Río Hernández, Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6(7), e352 (2017)
S. Fuchs, A.U. Ernst, L.-H. Wang, K. Shariati, X. Wang, Q. Liu, M. Ma, Hydrogels in emerging technologies for type 1 diabetes. Chem. Rev. 121(18), 11458 (2021)
C.H. Luu, G. Nguyen, T.-T. Le, T.-M.N. Nguyen, V.H. Giang Phan, M. Murugesan, R. Mathiyalagan, L. Jing, G. Janarthanan, D.C. Yang, Y. Li, T. Thambi, Graphene oxide-reinforced alginate hydrogel for controlled release of local anesthetics: synthesis, characterization, and release studies. Gels 8(4), 246 (2022)
M. Peruzynska, K. Cendrowski, M. Barylak, M. Tkacz, K. Piotrowska, M. Kurzawski, E. Mijowska, M. Drozdzik, Comparative in vitro study of single and four layer graphene oxide nanoflakes—Cytotoxicity and cellular uptake. Toxicol. In Vitro 41, 205 (2017)
G. Fontana, D. Thomas, E. Collin, A. Pandit, Microgel microenvironment primes adipose-derived stem cells towards an NP cells-like phenotype. Adv. Healthc. Mater. 3(12), 2012 (2014)
E. Garcia-Alegria, M. Iliut, M. Stefanska, C. Silva, S. Heeg, S.J. Kimber, V. Kouskoff, G. Lacaud, A. Vijayaraghavan, K. Batta, Graphene Oxide promotes embryonic stem cell differentiation to haematopoietic lineage. Sci. Rep. 6(1), 25917 (2016)
W.C. Lee, C.H.Y.X. Lim, H. Shi, L.A.L. Tang, Y. Wang, C.T. Lim, K.P. Loh, Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 5(9), 7334 (2011)
I. Lasocka, L. Szulc-Dąbrowska, M. Skibniewski, E. Skibniewska, K. Gregorczyk-Zboroch, I. Pasternak, M. Hubalek Kalbacova, Cytocompatibility of graphene monolayer and its impact on focal cell adhesion, mitochondrial morphology and activity in BALB/3T3 fibroblasts. Materials 14(3), 643 (2021)
W. Zhang, L. Yan, M. Li, R. Zhao, X. Yang, T. Ji, Z. Gu, J.-J. Yin, X. Gao, G. Nie, Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 237(2), 61 (2015)
A. Llacua, B.J. de Haan, S.A. Smink, P. de Vos, Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes: Extracellar Matrix Components. J. Biomed. Mater. Res. A 104(7), 1788 (2016)
L.A. Llacua, A. Hoek, B.J. de Haan, P. de Vos, Collagen type VI interaction improves human islet survival in immunoisolating microcapsules for treatment of diabetes. Islets 10(2), 60 (2018)
L.A. Llacua, M.M. Faas, P. de Vos, Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 61(6), 1261 (2018)
F. Menaa, A. Abdelghani, B. Menaa, Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: impact for tissue engineering and regenerative medicine: the G point in stem cell research? J. Tissue Eng. Regen. Med. 9(12), 1321 (2015)
A. Jarosz, M. Skoda, I. Dudek, D. Szukiewicz, Oxidative stress and mitochondrial activation as the main mechanisms underlying graphene toxicity against human cancer cells. Oxid. Med. Cell. Longev. 2016, 1 (2016)
T. Zhou, B. Zhang, P. Wei, Y. Du, H. Zhou, M. Yu, L. Yan, W. Zhang, G. Nie, C. Chen, Y. Tu, T. Wei, Energy metabolism analysis reveals the mechanism of inhibition of breast cancer cell metastasis by PEG-modified graphene oxide nanosheets. Biomaterials 35(37), 9833 (2014)
S.F. Kiew, L.V. Kiew, H.B. Lee, T. Imae, L.Y. Chung, Assessing biocompatibility of graphene oxide-based nanocarriers: a review. J. Controlled Release 226, 217 (2016)
N.H. McClenaghan, C.R. Barnett, E. Ah-Sing, Y.H.A. Abdel-Wahab, F.P.M. O’Harte, T.-W. Yoon, S.K. Swanston-Flatt, P.R. Flatt, Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11 produced by electrofusion. Diabetes 45(8), 1132 (1996)
H. Zhou, B. Zhang, J. Zheng, M. Yu, T. Zhou, K. Zhao, Y. Jia, X. Gao, C. Chen, T. Wei, The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials 35(5), 1597 (2014)
B. Zhang, P. Wei, Z. Zhou, T. Wei, Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 105, 145 (2016)
A. Acarregui, J. Ciriza, L. Saenz del Burgo, H. Gurruchaga Iribar, J. Yeste, X. Illa, G. Orive, R.M. Hernández, R. Villa, J.L. Pedraz, Characterization of an encapsulated insulin secreting human pancreatic beta cell line in a modular microfluidic device. J. Drug Target. 26(1), 36 (2018)
M. Zhang, S. Yan, X. Xu, T. Yu, Z. Guo, M. Ma, Y. Zhang, Z. Gu, Y. Feng, C. Du, M. Wan, K. Hu, X. Han, N. Gu, Three-dimensional cell-culture platform based on hydrogel with tunable microenvironmental properties to improve insulin-secreting function of MIN6 cells. Biomaterials 270, 120687 (2021)
O. Vargas, Á. Caballero, J. Morales, G.A. Elia, B. Scrosati, J. Hassoun, Electrochemical performance of a graphene nanosheets anode in a high voltage lithium-ion cell. Phys. Chem. Chem. Phys. 15(47), 20444 (2013)
Instituto Colombiano de Normas Técnicas y Certificación: (2011).
Z. Huang, L. Qian, Q. Yin, N. Yu, T. Liu, D. Tian, Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 66, 319 (2018)
F. Ganji, S. Vasheghani-Farahani, E. Vasheghani-Farahani, Theoretical description of hydrogel swelling: a review. Iran. Polym. J. 19(5), 375–398 (2010)
N. Ortiz-Romero, L.A. Ochoa-Martínez, S.M. González-Herrera, O.M. Rutiaga-Quiñones, J.A. Gallegos-Infante, Avances en las investigaciones sobre la encapsulación mediante gelación iónica: una revisión sistemática. TecnoLógicas 24(52), e1962 (2021)
Y. Sánchez-Cardona, C.E. Echeverri-Cuartas, M.E.L. López, N. Moreno-Castellanos, Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers 13(14), 2372 (2021)
Q.K. Anjani, A.H.B. Sabri, J. Domínguez-Robles, N. Moreno-Castellanos, E. Utomo, L.A.H. Wardoyo, E. Larrañeta, R.F. Donnelly, Metronidazole nanosuspension loaded dissolving microarray patches: an engineered composite pharmaceutical system for the treatment of skin and soft tissue infection. Biomater. Adv. 140, 213073 (2022)
M.R. Álvarez Santos, Y. Bueno Duarte, F.M. Güiza, A.R. Romero Bohórquez, S.C. Mendez-Sanchez, Effects of new tetrahydroquinoline-isoxazole hybrids on bioenergetics of hepatocarcinoma Hep-G2 cells and rat liver mitochondria. Chem. Biol. Interact. 302, 164 (2019)
R.L. Youngblood, J.P. Sampson, K.R. Lebioda, L.D. Shea, Microporous scaffolds support assembly and differentiation of pancreatic progenitors into β-cell clusters. Acta Biomater. 96, 111 (2019)
Acknowledgments
We thank Dr. Raquel Elvira Ocazionez of the Universidad Industrial de Santander, Colombia for allowing us to use her laboratories.
Funding
Open Access funding provided by Colombia Consortium. This research was supported by Universidad Industrial de Santander Project 2540. Finally, to the contributions of the international mobility programme for professors of the “Vicerrectoría de Investigación of the Universidad Industrial de Santander”, Colombia.
Author information
Authors and Affiliations
Contributions
Data curation, formal analysis, and methodology, MCV-R, AVR-S, EC-G, OV-C, and NM-C; funding acquisition and project administration, NM-C. Writing—original draft, EC-G, OV-C, and NM-C All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
The ethics committee of the Universidad Industrial de Santander approved this study (code4110) on May 10, 2019.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreno-Castellanos, N., Velásquez-Rincón, M.C., Rodríguez-Sanabria, A.V. et al. Encapsulation of beta-pancreatic cells in a hydrogel based on alginate and graphene oxide with high potential application in the diabetes treatment. Journal of Materials Research 38, 2823–2837 (2023). https://doi.org/10.1557/s43578-023-01009-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01009-6