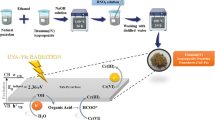
Abstract
Heterogeneous composites of metalloporphyrins with TiO2 possess exceptional photosensitizing properties, which works efficiently in degradation of organic pollutants. Herein, vanadium porphyrin complex {VO(TPP)} was loaded on TiO2 photocatalyst {VO(TPP)–TiO2} by the wet impregnation method. The as-prepared catalysts were characterized to analyze physicochemical properties and the photocatalytic degradation of 4-nitrophenol (4-NP) was investigated to assess their catalytic efficiency. XRD results revealed that VO(TPP)–TiO2 is dual-phase anatase/rutile with high crystallinity. The UV-DRS study showed that the loading of {VO(TPP)} on TiO2 caused a red-shift in the absorption spectrum and decreased the band-gap as well. The surface area has increased significantly after impregnation as confirmed by BET isotherm. The degradation of 4-NP follows pseudo-first-order kinetics, and the VO(TPP)–TiO2 showed higher degradation (99%) of 4-NP as compared to pure TiO2 (87%). The degradation mechanism of 4-NP and its pathway was also discussed using UV–Vis spectroscopy and electrospray mass spectrometry analysis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few decades, industrialization and anthropogenic activities have increased the catastrophic impact on the environment [1,2,3]. In water streams, the presence of hazardous compounds, such as phosphates, petroleum hydrocarbons, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, nitrophenols, etc., severely impacts the animal and the plant kingdom [4].
Nitrophenol (NP) is one of the leading water pollutants and enlisted among the top 114 toxic chemicals, as per US EPA. These chemicals were discharged from different industries like pesticides, medicines, dye, paper, textile, gas, and coke production industries, petrochemical industries [5,6,7]. Due to its high water solubility, stability, poisonous nature, and resistance towards biological treatments, its remediation becomes a serious issue for the scientific community. The pharmaceutical and dye industries are continuously using 4-nitrophenol (4-NP) as an intermediate reagent, despite its non-biodegradability and high toxicity [8, 9]. Thus, it is essential to treat the effluent containing nitro-phenolic compounds before their discharge into water bodies. In the last few years, numerous techniques have been employed for the degradation of nitro-phenolic compounds, such as oxidation, reduction [10, 11], microbial degradation [12], photocatalytic degradation [13], electrochemical method [14], adsorption [15], hydrogenation reactions [16], and Fenton process. However, most of these methods only transform the pollutant from one phase to another instead of complete removal. Therefore, further separation and purification techniques are required for their complete treatment before disposal. So, it becomes essential to develop a more efficient and economical method for treating nitrophenol in aqueous solution.
Heterogeneous photocatalysis has emerged as a promising alternative method for remediation of organic pollutants present in aqueous phase. It completely mineralizes organic contaminants without generating toxic intermediate compounds [17]. Hence, it has been considered as an efficient, eco-friendly, and low-cost green technique for wastewater decontamination [18]. The utilization of titanium dioxide (TiO2) semiconductor as a heterogeneous photocatalyst and photosensitizer has attracted the attention of the researchers. Its use is continuously increasing in the past two decades due to cost effectiveness, high stability, and non-toxicity [19]. The technological application of TiO2 is restricted due to its wide band-gap (3.0–3.2 eV), low photonic yield, and large electron–hole recombination rate [13]. Besides, it can only able to utilize 4% of solar spectrum by absorbing UV light of λ < 387 nm.
Researchers have explored various types of advanced oxidation technology to overcome the above mentioned limitations of TiO2. For example, Kim et al. studied the novel hybrid system of TiO2 photocatalyst for decomposition of non-biodegradable water contaminants. They observed that the decaying rate of pollutants is higher in the single-component system than in the mixed component system [20]. Recently, one research group synthesized TiO2 supported biochar by sol–gel method and used it for the treatment of reactive brilliant blue KN-R [21]. Likewise, Choi et al. developed a TiO2 membrane to treat water contamination [22]. Apart from water treatment, TiO2 is also utilized in some other applications, including air purification [23], photoelectrochemical water splitting [24], photo-conversion [11], and cement production [25]. Similar to TiO2, porphyrins also possess exceptional photosensitizing properties due to the presence of grand π-electron systems, macrocyclic conjugate structure, thermal stability, and high absorption solar radiation coefficient (400–450 nm region and 500–700 nm region) [26]. According to the literature, TiO2 in combination with porphyrins can efficiently degrade the organic pollutant molecules in water by employing solar radiation [27, 28]. Porphyrins facilitate the transfer of electrons to the conduction band of TiO2, increase adsorption of oxygen, and direct oxidation [29]. Heterogeneous composites of metalloporphyrins with TiO2 were also employed for hydrogen evolution through photocatalysis [30].
To the best of our knowledge, no study has been published on the removal of 4-nitrophenol using vanadium porphyrin-loaded TiO2 {VO(TPP)–TiO2} photocatalyst. Thus in the current work, a vanadium porphyrin was impregnated on pure TiO2 photocatalyst, and its photocatalytic activity was assessed through degradation of 4-NP. The photocatalysts were characterized for their physicochemical properties using various characterization tools like X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), BET surface area, UV–Vis diffuse reflectance spectroscopy (UV–Vis DRS), and scanning electron microscope (SEM)- energy-dispersive X-ray (EDX). The optimization studies, including UV light irradiation time, catalyst dose, and variation in 4-NP concentration, were carried out to compare the efficiency of pure TiO2 and VO(TPP)–TiO2. The degradation mechanism and kinetics involved are also discussed to elucidate the degradation pathway and rate of degradation reaction.
Results and discussion
The XRD patterns of pure TiO2 and VO(TPP)–TiO2 are shown in Fig. 1. Pure TiO2 shows diffraction peaks at 25.42°, 37.86°,48.09°, 53.96°, 55.28°, and 63.07° (PDF 86-1157), which corresponds to (101), (004), (200), (105), (211), and (204) planes of pure anatase phase. After impregnation with VO(TPP), a new peak emerges at 28.51° (PDF 86-0147), corresponding to the (110) plane of the rutile phase of TiO2. This shows that the VO(TPP)–TiO2 is composed of both the rutile and anatase phases of TiO2. VO(TPP)–TiO2 contains a mixture of 90% anatase and 10% rutile phase with a trace amount of brookite (peak at 30.81°). From the Fig. 1, it can be clearly seen that after impregnation, the crystallinity of the catalyst increased, confirmed by the appearance of sharp peaks.
It can be seen in Table 1 that after impregnation, there is an increment in particle size of 0.01 nm. The rutile and brookite phase exhibits high stability when the particle size lies between 15 and 34 nm [31]. On the other hand, the anatase phase shows more stability in the crystal lattice when the particle size is < 14 nm. The anatase particles undergo coagulation and agglomeration during impregnation to form large particles (17.13 nm). These large particles distort the crystal stability, and thus they transform into the most stable rutile phase at such a larger particle size.
Figure 2 shows the FTIR spectra of TiO2 and VO(TPP)–TiO2. The broad band at around 3400 to 3500 cm−1 corresponds to the stretching vibration of the –OH group of TiO2. A band observed at 1628 cm−1 belongs to the bending mode of H–O–H [32]. A peak at around 468 cm−1 represents bonds due to Ti–O and Ti–O–Ti linkage [33]. After impregnation with VO(TPP), new bands appear at 2915 and 2851 cm−1, which corresponds to the C–H asymmetric and symmetric stretching vibrations. A weak band around 1007 cm−1can be attributed to the pyrrole ring stretching. The band at around 601 cm−1 has also emerged in VO(TPP)–TiO2, which corresponds to the V–O bond [34].
The low-temperature N2 adsorption–desorption isotherm of pure TiO2 and VO(TPP)–TiO2 is shown in Fig. 3. An isotherm of type IV with an H1-type hysteresis loop signifying open-ended cylindrical pores is obtained. The isotherm shows a characteristic of mesoporous materials. The insertion of metal-porphyrin on the TiO2 significantly influences the surface area of TiO2. It is observed that the surface area of VO(TPP)–TiO2 is 175.14 m2/g in comparison to pure TiO2 137.85 m2/g. The inset in Fig. 3 shows the pore-size distribution, containing mesopores with a pore diameter in the range of 3–30 nm.
The absorption spectra of TiO2, VO(TPP), and VO(TPP)–TiO2 obtained by UV–Vis diffuse reflectance spectroscopy are shown in Fig. 4. The spectrum of pure TiO2 reveals that absorption started around 390 nm in the UV region. The VO(TPP) spectrum showed Soret band at 411 nm, appears due to transition from a1u (π) to eg* (π) and Q-bands at 529 nm, 580 nm, 630 nm, and 640 nm, respectively, emerge from a2u (π) to eg* (π) transition [35]. The impregnation with TiO2 (VO(TPP)–TiO2) induces the red-shift of the Soret band from 411 to 432 nm and a single Q band appears at 546 nm. The band-gap (Eg) of TiO2 was evaluated using Tauc's equation [36] and baseline approach [37] was used to calculate the Eg of VO(TPP)–TiO2. The Eg values were slightly reduced from 3.20 to 3.19 eV for pure TiO2 and VO(TPP)–TiO2, respectively. Compared to pure TiO2, VO(TPP)–TiO2 showed enhanced visible light absorption and a slight decrease in Eg. The red-shift in absorption facilitates more absorption of photons by the catalysts and thus improves light utilizing efficiency [38]. The reduction in the Eg values (Fig SI 1b) occurred due to the presence of vanadium porphyrin, which forms a junction between the valence band (VB) and conduction band (CB) of TiO2. This junction facilitates the rapid transfer of electrons generated by vanadium porphyrin to the CB of TiO2 and thus reduces the forbidden bandwidth.
The SEM images shown in figure SI 2 indicate that the VO(TPP)–TiO2 sample (Fig. SI 2a) has more uneven-shaped particles than the pure TiO2 (Fig. SI 2c). The regular and smooth surface is observed in the pure TiO2 sample. The slightly bright surface can also be noticed in the VO(TPP)–TiO2, which shows the presence of functionalized moiety on the surface of TiO2. Simultaneously, the particle size of porphyrin-loaded TiO2 in aggregates is smaller than the pure TiO2. The EDX analysis gives quantitative information about the elemental composition. The presence of vanadium (Fig. SI 2b) indicates the impregnation of the porphyrin complex on the surface of TiO2. Further, the increase in wt% of carbon and decrease in wt% of Ti also confirms the impregnation of vanadium porphyrin.
Photocatalytic degradation (PCD) studies
TiO2, VO(TPP), and VO(TPP)–TiO2 catalysts photocatalytic efficiency was examined by conducting photocatalytic degradation experiments with 4-NP. Various parameters like UV light irradiation, the dose of catalyst, and the effect of initial pollutant concentration (4-NP) were examined. Figure 5(a) illustrates the effect of surface modification of TiO2 using V- porphyrin complex on the PCD of 4-NP. It is clear from the figure that VO(TPP) is photocatalytic inactive catalyst and therefore the 4-NP solution concentration remains same after 180 min of UV light illumination. However, the catalytic efficiency of VO(TPP)–TiO2 to degrade 4-NP is greater than TiO2 after 180 min of UV light irradiation. Initially, in all PCD experiments, the reaction was performed in the absence of UV light (dark condition) for 30 min to examine the adsorption efficiency of the photocatalyst. After that, the influence of duration of UV light illumination (0–180 min) on the degradation efficiency of a photocatalyst for the 4-NP solution was monitored. It was observed that the 4-NP degradation get increased with enhancement of light illumination duration. However, during the initial 60 min, the normalized concentration (C/C0) of 4-NP decreased rapidly. After that, the degradation slows down with irradiation time. This effect is more prominent in the case of TiO2. Thus, all the PCD experiments were carried out for the optimized duration, i.e., 180 min.
Influence of (a) illumination time on PCD of 4-NP in the presence of VO(TPP), TiO2 and VO(TPP)-TiO2 {(4-NP conc. = 5 ppm; catalyst dose = 1.5 g/Lt)}, (b) catalyst dose on PCD of 4-NP (5 ppm), (c) initial concentration of 4-NP on its PCD {catalyst dose for (TiO2 = 1.0 g/Lt and VO(TPP)–TiO2 = 1.5 g/Lt)}, (d) pseudo-first-order rate plot of PCD of 4-NP under UV irradiation with VO(TPP)–TiO2 {Dose of VO(TPP)-TiO2 = 1.5 g/Lt}.
The role of catalyst’s dose is one of the deciding factors in determining the maximum degradation efficiency of a photocatalyst. The PCD experiments of 4-NP at a fixed concentration (5 ppm) were conducted with variable doses (0.1–2.5 g/L) of TiO2 and VO(TPP)–TiO2. Figure 5(b) shows the PCD of 4-NP after 180 min with different catalyst doses. In TiO2, the PCD increases with the addition of photocatalyst dose until 1.0 g/L, and after that, the degradation reduces up to a dose of 2.5 g/L. Similarly, in the case of VO(TPP)–TiO2, the PCD increases up to 1.5 g/L dose and then decreases even with the increase in dose up to 2.5 g/L. The initial increment in PCD is due to the generation of many active sites and free radicals (hydroxyl and superoxide radicals) [39]. Whereas, increase in photocatalyst dose (more than 1.0/1.5 g/L) obstructs the path of light which falls into the 4-NP solution and may also cause turbidity. More photocatalyst particles hindered the light path and thus inhibited the process of radical’s generation, which ultimately reduced the PCD [40]. Thus, TiO2 showed maximum degradation of 4-NP at 1.0 g/L dose, whereas VO(TPP)–TiO2 optimized dose was 1.5 g/L for maximum degradation of 4-NP, which is more significant in comparison to TiO2. The increase in photocatalytic efficiency of VO(TPP)–TiO2 can be explained based on the effectiveness of this material in the separation of the charge carriers (electron–hole pairs), which is generated during illumination [41, 42].
The influence of 4-NP concentration (from 1 to 20 ppm) on the PCD of TiO2 and VO(TPP)–TiO2 was also assessed. Figure 5(c) depicts the variation in PCD with the change in 4-NP concentration in the presence of TiO2 and VO(TPP)–TiO2 photocatalyst. Both the photocatalyst [TiO2 and {VO(TPP)–TiO2}] showed a similar trend of decrease in PCD with an increase in initial 4-NP concentration. The generation and the number of radicals (.OH and.O2−) and holes available at a particular fixed dose of catalyst are limited during the irradiation duration. As well as, the rate of emission of photons by UV irradiation is steady. Nevertheless, the concentration of 4-NP molecules increases, which is exceeded in comparison to the availability of radicals for oxidation [43]. Additionally, in a PCD reaction, the adsorption of by-products and intermediates generated via the oxidation process of 4-NP onto the active sites might cause deactivation. Due to this reason, the PCD decreases [21] with an increase in the initial concentration of 4-NP.
For determining the PCD reaction rate, pseudo-first-order kinetics (1) was applied to the obtained kinetic results.
where k is the first-order rate constant (min−1), C0 represents the initial concentration of 4-NP in ppm; C represents the remaining concentration of 4-NP in ppm after time t (min) under illumination with UV light. The pseudo-first-order kinetic plot of degradation of the 4-NP in presence of VO(TPP)–TiO2 photocatalyst is presented in Fig. 5(d). The k value was determined by the slope of an obtained straight line of ln(C0/C) vs. t plot. From the graph, it is inferred the rate of PCD reaction decreased with an increase in the concentration of 4-NP. Because, the k values calculated from the slope of straight line are 0.030 min−1, 0.012 min−1, 0.006 min1, 0.005 min−1, and 0.003 min−1 for 1, 5, 10, 15, and 20 ppm 4-NP, respectively. Thus, the pseudo-first-order kinetics data (Table SI 1) confirms that the less concentrated solution of 4-NP is degraded faster than the highly concentrated one.
Degradation mechanism of 4-NP
Figure 6 represents the changes in absorption spectra of 4-NP solution with time during PCD reaction in the presence of VO(TPP)–TiO2. These changes in 4-NP were monitored using a UV–Vis spectrophotometer. Significant changes are observed from the UV–Vis spectra of 4-NP solution aliquots containing their different concentrations at different time intervals. Un-degraded 4-NP (initial) absorption spectrum exhibited a characteristic peak at λmax 316 nm, shown in Fig. 6. After illumination with UV light (15–30 min), the intensity of this peak gets decreased. Moreover, also a new peak has emerged at about 410 nm, corresponding to p-nitrocatechol [44]. Then, after irradiation of about 60 min, this absorption band disappears along with the 316 nm characteristic peak. Thus, it is clear from the spectral change that 1 ppm [Fig. 6(a)] and 5 ppm [Fig. 6(b)] 4-NP after 180 min of exposure to UV light, complete mineralization into carbon dioxide and water molecules has occurred in the presence of VO(TPP)–TiO2 [17]. But, in the case of a higher concentration of 4-NP (10 ppm), complete mineralization does not occur. However, after 180 min of illumination, the 4-NP solution concentration becomes decreased to less than half of the initial concentration (Fig. 6c). Further, to identify the degradation products and the mechanism involved, electrospray mass spectrometry (ESMS) analysis was performed. The ESMS -spectrum is shown in Fig. 7 and was recorded at positive mode. The possible structure of intermediates and the proposed mechanism of 4-NP is shown in Scheme 1, which is in good agreement with a previous report [44]. In this mechanism, initially, OH radicals react on 4-NP at ortho position with respect to the hydroxyl group and thus form nitro-catechol (VI, m/z = 153) via pathway (i). In the second pathway (ii), hydroxylation of 4-NP generated a benzoquinone (II, m/z = 110) intermediate. The nitro-catechol lose the nitro group and form hydroxyquinol (VII, m/z = 125.9). Then both the generated intermediates were oxidized to low molecular weight saturated [propane-dioic acid (IV, m/z = 104) and oxalic acid (V)] and unsaturated carboxylic acids [but-2-enedioic acid (III, m/z = 141)].
Conclusions
In conclusion, a photocatalyst was prepared by impregnating VO(TPP) on TiO2 and characterized by various characterization techniques. The band-gap was reduced to 3.19 eV in VO(TPP)–TiO2 compared to 3.2 eV in TiO2. The XRD result showed that VO(TPP)–TiO2 is a mixed-phase catalyst containing anatase and rutile phases. The photocatalytic activity of pure and VO(TPP)–TiO2 was assessed based on the % degradation of 4-NP. The optimization studies showed that the maximum degradation of 4-NP was achieved at 180 min irradiation time under UV light. The dose of 1.0 g/L and 1.5 g/L of TiO2 and VO(TPP)–TiO2, respectively, showed maximum degradation of 4-NP. The extent of degradation of 4-NP gets decreased with an increase in its initial concentration with VO(TPP)–TiO2. However, this effect is more pronounced when 4-NP concentration increases above 5 ppm, as evidenced by pseudo-first-order rate constant values. Although, VO(TPP)–TiO2 showed 99% degradation of 4-NP solution of 1 and 5 ppm concentration. The degradation pathway deduced on the basis of UV–Vis and ESMS spectra confirmed that the 4-NP completely oxidized into carbon dioxide and water molecules in the presence of VO(TPP)–TiO2 and UV light. This study suggested that the impregnation of metal-porphyrin on a photocatalyst surface is an efficient surface approach and may be explored for the degradation of other emerging water contaminants from industrial wastewater.
Material and methods
Materials
Titanium dioxide (TiO2) used in this work, was synthesized through a sol–gel method reported in our previous work [13]. Briefly, titanium isopropoxide (TTIP-8 ml) was dissolved in ethanol (42 ml), to which a 1:1 solution of ethanol (50 ml) and water (50 ml) was mixed drop-wise at 80 °C. The resultant solution was stirred for another 60 min and then aged for 24 h at room temperature. The solution having white precipitate was then placed in an oven at 120 °C for 12 h (or till it was completely dry). Eventually, the dried product was manually grinded and annealed for 4 h at 400 °C in the air with a heating ramp of 3 °C/min. The other chemicals and solvents such as, 5,10,15,20-tetraphenyl-21H,23H-porphyrin vanadium oxide {VO(TPP)}, ethanol, and dichloromethane (DCM) were purchased from Sigma-Aldrich. The 4-NP was obtained from Rankem (95% purity). In all the experiments, Milli-Q water was used.
Preparation of VO(TPP)–TiO2
The metal-loaded porphyrin-titanium dioxide {VO(TPP)–TiO2} was prepared by the wet impregnation method. Firstly, 5 g of TiO2 powder was suspended in 20 ml DCM solvent. Then, 3.5 mg of 5,10,15,20-tetraphenyl-21H,23H-porphyrin vanadium oxide (VO(TPP)) was dissolved in 5 ml DCM, and added to the suspended TiO2 solution. After that the mixture was stirred for 5 h at 50 °C. Then, the product was segregated by evaporating the solvent in a vacuum. The light pinkish product was obtained after drying in an oven at 40 °C for 2 h. Finally, it was calcined at 350 °C for 4 h and a loading % of 0.07 (VO(TPP)) on TiO2 was measured.
Characterization
The prepared catalyst's X-ray diffraction (XRD) pattern was recorded on Bruker AXSD8 diffractometer with the copper radiation (Cu-Kα) of wavelength 1.54 Ǻ as an X-ray source. All the photocatalysts were scanned in 2θ mode and analyzed in the range of 20°–80°. The crystallite sizes of the samples were determined using following Debye–Scherrer Eq. 2 [45]:
where, K = 0.89 is shape factor, λ is the wavelength of X-ray irradiation, Cu-Kα of wavelength 1.54 Ǻ, β is the full-width half-maximum (FWHM) of the diffraction peak, and θ is the X-ray diffraction angle.
The ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis DRS) of all the photocatalysts was performed on Shimadzu UV-2550 spectrophotometer and scanned in the range of 200–800 nm wavelength. The spectrophotometer was equipped with an integrating sphere assembly and barium sulphate (BaSO4) was employed as a reference standard. The morphology and elemental mapping of the prepared catalyst was obtained through Scanning electron microscopy (SEM) equipped with the energy-dispersive X-ray (EDX) on FEI Quanta 200 F. The Brunauer–Emmett–Teller (BET) surface area of the catalysts was determined using Micromeritics ASAP 2020 model surface area analyzer. Prior to analysis, all the samples were degassed for 4 h at 350 °C. The pore-size distribution (pore diameter and pore volume of samples) was determined by the Barrett-Joyner-Halenda (BJH) method. The Fourier transform infrared (FTIR) spectra of the photocatalysts were recorded in transmission mode using a Perkin Elmer spectrometer. The catalyst powders were mixed with potassium bromide (KBr) to form thin transparent pellets. Then, the spectra of the pellets containing catalyst were recorded in the wavenumber range of 4000–400 cm−1. The electrospray mass spectrometry (ESMS) analysis was performed on Maldi Synapt XS HD Mass Spectrometer in a positive mode to obtain the m/z ratios of intermediates and degradation products involved in the degradation pathway of 4-NP.
Photocatalytic degradation experiments
The photocatalytic activity of photocatalysts was evaluated through the degradation of 4-NP in a photocatalytic batch reactor (Lelesil Innovative Systems Ltd. Mumbai, India), containing a 125-W mercury lamp for UV light irradiation (λ ≈ 354 nm). The lamp was enclosed in a cylindrical vessel (500 ml) made up of quartz. A double-walled jacket around the lamp circulates the water to maintain the temperature.
A typical photocatalytic experiment was performed using a 150 ml solution of 4-NP in a pre-determined concentration. A required amount of catalyst was added to the solution and stirred for 30 min without light to reach adsorption equilibrium. Then, the reaction solution was illuminated with UV light under continuous stirring. The aliquots (5 ml) were taken out at different time intervals up to 3 h and were filtered through a syringe filter (Millipore, 0.45 µm) to remove the photocatalyst. The remaining concentration of 4-NP in filtered solution was determined by measuring the absorbance through UV–Vis spectrophotometer (Thermo Scientific, Evolution 201) at λmax = 316 nm.
The photocatalytic batch experiments were carried out in duplicates wherever required, and some of the experiments were repeated up to three times. The uncertainty of the results from experiments was estimated by the repeatability of the experiments, which was found below ± 2% and had no significant influence on the interpretation of the results.
Data availability
Not available.
Code availability
Not available.
References
J. Markus, A.B. McBratney, A review of the contamination of soil with lead II. Spatial distribution and risk assessment of soil lead. Environ. Int. 27(5), 399–411 (2001)
M. Maisonet, A. Correa, D. Misra, J.J.K. Jaakkola, A review of the literature on the effects of ambient air pollution on fetal growth. Environ. Res. 95(1), 106–115 (2004)
T. Gao, H. Chen, S. Xia, Z. Zhou, Review of water pollution control in China. Front. Environ. Sci. Eng. China 2(2), 142–149 (2008)
S. Rasalingam, R. Peng, R.T. Koodali, Removal of hazardous pollutants from waste waters: applications of TiO2–SiO2 mixed oxide materials. Nanomaterial. (2014). https://doi.org/10.1155/2014/617405
K. Zhang, Y. Liu, J. Deng, S. Xie, H. Lin, X. Zhao, J. Yang, Z. Han, H. Dai, Fe2O3/3DOM BiVO4: high-performance photocatalysts for the visible light-driven degradation of 4-nitrophenol. Appl. Catal. B Environ. 202, 569–579 (2017)
M.I. Din, R. Khalid, Z. Hussain, T. Hussain, A. Mujahid, J. Najeeb, F. Izhar, Nanocatalytic assemblies for catalytic reduction of nitrophenols: a critical review. Crit. Rev. Anal. Chem. 50(4), 322–338 (2020)
J. Yang, B. Pan, H. Li, S. Liao, D. Zhang, M. Wu, B. Xing, Degradation of p-nitrophenol on biochars: role of persistent free radicals. Environ. Sci. Technol. 50(2), 694–700 (2016)
P. Gharbani, M. Khosravi, S.M. Tabatabaii, K. Zare, S. Dastmalchi, A. Mehrizad, Degradation of trace aqueous 4-chloro-2-nitrophenol occurring in pharmaceutical industrial wastewater by ozone. Int. J. Environ. Sci. Technol. 7(2), 377–384 (2010)
M. Ismail, M.I. Khan, S.B. Khan, M.A. Khan, K. Akhtar, A.M. Asiri, Green synthesis of plant supported Cu–Ag and Cu–Ni bimetallic nanoparticles in the reduction of nitrophenols and organic dyes for water treatment. J. Mol. Liq. 260, 78–91 (2018)
R.K. Narayanan, S.J. Devaki, Brawny silver-hydrogel based nanocatalyst for reduction of nitrophenols: studies on kinetics and mechanism Ind. Eng. Chem. Res. 54(4), 1197–1203 (2015)
D. Kim, A. Ghicov, P. Schmuki, TiO2 Nanotube arrays: elimination of disordered top layers (“nanograss”) for improved photoconversion efficiency in dye-sensitized solar cells. Electrochem. Commun. 10(12), 1835–1838 (2008)
Y. Dai, R. Chen, J. Lin, J. Xiao, S. Chen, S. Deng, Reduction of nitro phenols using nitroreductase from E. coli in the presence of NADH. J. Hazard. Mater. 170(1), 141–143 (2009)
V. Yadav, P. Verma, H. Sharma, S. Tripathy, V.K. Saini, Photodegradation of 4-nitrophenol over B-doped TiO2 nanostructure: effect of dopant concentration, kinetics, and mechanism. Environ. Sci. Pollut. Res. 27(10), 10966–10980 (2020)
S. Singh, N. Kumar, M. Kumar, Jyoti, A. Agarwal, B. Mizaikoff, Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem. Eng. J. 313, 283–292 (2017)
H.S. Kim, D. Kim, B.S. Kwak, G.B. Han, M.H. Um, Synthesis of magnetically separable core at shell structured NiFe2O4 at TiO2 nanomaterial and its use for photocatalytic hydrogen production by methanol/water splitting. Chem. Eng. J. 243, 272–279 (2014)
Z. Wu, J. Chen, Q. Di, M. Zhang, Size-controlled synthesis of a supported Ni nanoparticle catalyst for selective hydrogenation of P-nitrophenol to P-aminophenol. Catal. Commun. 18, 55–59 (2012)
E. Di Paola, A. Augugliaro, V. Palmisano, L. Pantaleo, G. Savinov, Heterogeneous photocatalytic degradation of nitrophenols. J. Photochem. Photobiol. A Chem. 155(1–3), 207–214 (2003)
O.A. Osin, T. Yu, S. Lin, Oil refinery wastewater treatment in the Niger Delta, Nigeria: current practices, challenges, and recommendations. Environ. Sci. Pollut. Res. 24(28), 22730–22740 (2017)
A.L. Linsebigler, G. Lu, J.T. Yates, Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95(3), 735–758 (1995)
S.J. Kim, S.C. Kim, S.G. Seo, D.J. Lee, H. Lee, S.H. Park, S.C. Jung, Photocatalyzed destruction of organic dyes using microwave/UV/O3/H2O2/TiO2 oxidation system. Catal. Today. 164(1), 384–390 (2011)
S. Zhang, X. Lu, Treatment of wastewater containing reactive brilliant blue KN-R using TiO2/BC composite as heterogeneous photocatalyst and adsorbent. Chemosphere 206, 777–783 (2018)
H. Choi, M.G. Antoniou, A.A. de la Cruz, E. Stathatos, D.D. Dionysiou, Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 202(1–3), 199–206 (2007)
T.S. Le, T.H. Dao, D.C. Nguyen, H.C. Nguyen, I.L. Balikhin, Air purification equipment combining a filter coated by silver nanoparticles with a nano-TiO2 photocatalyst for use in hospitals. Adv. Nat. Sci. Nanosci. Nanotechnol. 6(1), 15016 (2015)
Y. Zhongrui, L. Ying, Q. Jiangtao, Z. Rongkun, C.M. Julie, Z. Mingyuan, K. Aslam, L. Wenxian, Z. Jiujun, Enhanced photoelectrochemical water-splitting performance with a hierarchical heterostructure: CO3O4 nanodots anchored TiO2@P-C3N4 coreshell nanorod arrays. Chem. Eng. J. 404, 126458 (2021)
M.J. Gazquez, J.P. Bolivar, F. Vaca, R. García-Tenorio, A. Caparros, Evaluation of the use of TiO2 industry red gypsum waste in cement production. Cem. Concr. Compos. 37, 76–81 (2013)
J. Zhang, A. Wang, W. Zhao, C. Li, X. Chen, Y. Wang, W. Zhu, Q. Zhong, Influence of metal-porphyrins on the photocatalysis of graphitic carbon nitride. Dye. Pigment. 153, 241–247 (2018)
G. Mele, R. Del Sole, G. Vasapollo, G. Marcì, E. Garcìa-Lòpez, L. Palmisano, J.M. Coronado, M.D. Hernández-Alonso, C. Malitesta, M.R. Guascito, TRMC, XPS, and EPR characterizations of polycrystalline TiO2 porphyrin impregnated powders and their catalytic activity for 4-nitrophenol photodegradation in aqueous suspension. J. Phys. Chem. B 109(25), 12347–12352 (2005)
G. Mele, R. Del Sole, G. Vasapollo, E. García-López, L. Palmisano, L. Jun, R. Słota, G. Dyrda, TiO2-based photocatalysts impregnated with metallo-porphyrins employed for degradation of 4-nitrophenol in aqueous solutions: role of metal and macrocycle. Res. Chem. Intermed. 33(3–5), 433–448 (2007)
C. Wang, J. Li, G. Mele, G.M. Yang, F.X. Zhang, L. Palmisano, G. Vasapollo, Efficient degradation of 4-nitrophenol by using functionalized porphyrin-TiO2 photocatalysts under visible irradiation. Appl. Catal. B Environ. 76(3–4), 218–226 (2007)
G. Riyue, L. Xiangqing, Z. Bing, K. Shi-Zhao, Q. Lixia, L. Guodong, Cu2O/tetrakis (4-hydroxyphenyl)porphyrin hybrid with visible photocatalytic activity for hydrogen evolution. Appl. Catal. B Environ. 211(211), 296–304 (2017)
H. Zhang, J.F. Banfield, Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. Chem. Rev. 114(19), 9613–9644 (2014)
A. León, P. Reuquen, C. Garín, R. Segura, P. Vargas, P. Zapata, P.A. Orihuela, FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Applied Sciences. Appl. Sci. 7(1), 1–9 (2017)
C.H. Chen, S.S. His, H.C. Nian, S.H. Chiu, Synthesis of N-doped TiO2 photocatalyst for low-concentration elemental mercury removal under various gas conditions. Appl. Catal. B Environ. 160–161, 558–565 (2014)
A. Bellifa, D. Lahcene, Y.N. Tchenar, A. Choukchou-Braham, R. Bachir, S. Bedrane, C. Kappenstein, Preparation and characterization of 20 wt% V2O5-TiO2 catalyst oxidation of cyclohexane. Appl. Catal. A Gen. 305(1), 1–6 (2006)
L. Wenxian, H. Xiaofang, G. Riyue, Z. Mingyuan, F. Lingyan, L. Ying, Cobalt porphyrin (CoTCPP) advanced visible light response of g-C3N4 nanosheets. Sustain. Mater. Technol. 22, e00114 (2019)
J. Tauc, Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 5(8), 721–729 (1970)
M. Patrycja, P. Michał, M. Wojciech, How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 9(23), 6814–6817 (2018)
Y. Zhang, J. Xu, J. Mei, S. Sarina, Z. Wu, T. Liao, C. Yan, Z. Sun, Strongly interfacial-coupled 2D–2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 394, 122529 (2020)
L. Rizzo, D. Sannino, V. Vaiano, O. Sacco, A. Scarpa, D. Pietrogiacomi, Effect of solar simulated N-doped TiO2 photocatalysis on the inactivation and antibiotic resistance of an E. coli strain in biologically treated urban wastewater. Appl. Catal. B Environ. 144, 369–378 (2014)
H.S. Wahab, A.A. Hussain, Photocatalytic oxidation of phenol red onto nanocrystalline TiO2 particles. J. Nanostructure Chem. 6(3), 261–274 (2016)
M. Quesada-González, K. Baba, C. Sotelo-Vázquez, P. Choquet, C.J. Carmalt, I.P. Parkin, N.D. Boscher, Interstitial boron-doped anatase TiO2 thin-films on optical fibres: atmospheric pressure-plasma enhanced chemical vapour deposition as the key for functional oxide coatings on temperature-sensitive substrates. J. Mater. Chem. A 5(22), 10836–10842 (2017)
J. Gomes, J. Lincho, E. Domingues, R.M. Quinta-Ferreira, R.C. Martins, N-TiO2 photocatalysts: a review of their characteristics and capacity for emerging contaminants removal. Water 11(2), 373 (2019)
M.J.N. Gotostos, C.C. Su, M.D.G. De Luna, M.C. Lu, Kinetic study of acetaminophen degradation by visible light photocatalysis. J. Environ. Sci. Heal. Part A 49(8), 892–899 (2014)
E. Moctezuma, E. Leyva, C.A. Aguilar, R.A. Luna, C. Montalvo, Photocatalytic degradation of paracetamol: intermediates and total reaction mechanism. J. Hazard. Mater. 243, 130–138 (2012)
P. Scherrer, Göttinger Nachrichten. Math. Phys. 2, 98–100 (1918)
Acknowledgments
Vandana Yadav is thankful to University Grants Commission for her doctoral grant (NFO-2015-17-OBC-UTT-29056), which provided the impetus needed to carry out this work.
Funding
University Grants Commission (Grant No:NFO-2015-17-OBC-UTT-29056), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yadav, V., Verma, P., Negi, H. et al. Efficient degradation of 4-nitrophenol using VO(TPP) impregnated TiO2 photocatalyst: Insight into kinetics and mechanism. Journal of Materials Research 38, 237–247 (2023). https://doi.org/10.1557/s43578-022-00856-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00856-z