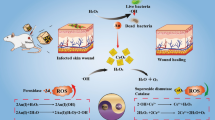
Abstract
In this work, functionalized reduced graphene oxide–silver (FrGO–Ag) nanocomposite was synthesized to enhance the antimicrobial activity and biocompatibility of FrGO for infected wound burn treatment. The reduction of GO and FGO was confirmed by the removal of some of the oxygen functional groups (carbonyl and epoxy groups) as revealed by Fourier transform infrared (FTIR) spectra. The face-centered cubic (fcc) silver nanoparticles were identified by X-ray diffraction (XRD). The contact time effect and the dose effect of the antimicrobial activity of rGO, FrGO, and FrGO-Ag nanocomposite toward Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans have been investigated. The cytotoxicity results of these compounds revealed that the functionalization by PVP and the decoration by AgNPs improved the biocompatibility of rGO sheets from 35.2 to 88% cell viability against the BJ1 normal human epithelial cell line.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graphene is the thinnest two-dimensional crystalline material that is steady at room temperature. It is a layer of sp2-bonded carbon atoms, organized to form honey comb crystal lattice, and is a 0.345 nm thick [1,2,3]. These carbon atoms are arranged in a hexagonal lattice with a C–C distance of 0.142 nm [1, 4]. It is considered the basic building block of all graphitic materials; such as fullerenes (zero dimensional (0D), nanotubes (1D), and graphite (3D) [5]. Graphene remarkable properties are reported as high carrier transport mobility as its mobility of charge carriers is ~ 200,000 cm2/V/s [6], superior mechanical flexibility with Young’s modulus is approximately 1100 GPa and fracture strength is 125 GPa [7], excellent thermal/chemical stability [8], optical transparency [9], high specific surface area with a calculated value of 2630 m2/g [10], excellent electrical and thermal conductivity of ~ 5000 W/m/K [11]. Chemical conversion, which involves sonicating flake graphite (Gt) to obtain graphene oxide (GO) and then reducing, allows for low-cost synthesis and processing of bulk-quantity graphene nanosheets, but the use of highly toxic and dangerously unstable NaBH4 or hydrazine to reduce GO necessitates extreme caution [12]. Another issue is the tendency of products to irreversible aggregation or restacking into Gt due to van der Waals interaction during the chemical reduction [13].
Functionalization has drawn attention since it solved the problem of graphene affinity toward aggregation. The chemical, electrochemical, and sono-chemical methods are examples of graphene functionalization, which is an essential step for preparing FrGO from rGO [14, 15]. There are two fundamental types of functionalization; covalent and non-covalent functionalization. Noncovalent interactions are primarily mediated by hydrophobic, van der Waals, and electrostatic forces and necessitate the physical adsorption of appropriate molecules on the graphene surface. Covalent functionalization is considered a structural alteration that can take place at the end of the graphene sheets and/or on their surface. There are four methods for covalent modification of graphene: nucleophilic substitution, electrophilic addition, condensation, and addition [14].
Polyvinylpyrrolidone (PVP) is a biocompatible linear polymer featuring hydrophilic and lipophilic groups in its backbone [16,17,18]. Recently, PVP reduced GO (FrGO) sheets have been studied for different applications, such as electroanalysis [19], microwave absorbing applications [20], and wastewater treatment [21]. In addition, the electrical [16], mechanical [16, 22], anticorrosive properties [23], and thermal conductivity [22] have been examined and reported. In wound dressing application, several articles have been reported. Contardi et al. [24] designed electrospun fibrous PVP based hydrogels loaded with hydroxycinnamic acids as potential wound dressings. Contardi et al. [25] fabricated a transparent PVP/hyaluronic acid-based bilayer for the sequential release and delivery of a cutaneous antiseptic and a widely used antibiotic, potentially suitable for wound dressing applications. Paul et al. [26] synthesized biodegradable, stretchable, and transparent plastic films by hybridizing a waterborne aliphatic polyester polyurethane (PU) suspension with PVP. This product has a wide range of applications including agricultural mulches, food and pharmaceutical packaging, and biomedical applications.
FrGO has been synthesized by different methods for various applications. Lee et al. [27] used Prussian blue-laden PVP/reduced pollutants for the removal of hazardous pollutants. Pavinatto et al. [28] prepared PVP/chitosan/rGO electrospun nanofibers to study the ultrasensitive biosensor [28]. Huang et al. [29] prepared PVP-assisted growth of Ni–Co oxide on N-doped rGO to enhance its pseudocapacitive behavior [29]. Li et al. [30] synthesized FeSO4/rGO/PVP ternary composite to enhance its microwave absorbing properties [30]. Despite all the reported studies, no one has reported the antimicrobial activity and the cytotoxicity of FrGO (PVP–rGO).
The biological applications of graphene-based materials have been enormously investigated because of their biocompatibility, unique conjugated structure, and relatively low cost [31]. The biological applications of graphene, such as DNA-hybridization device, cancer treatment [32], and delivery of drugs, have also started to be concerned [33]. It also shows a huge potential in other fields of the biological applications such as antibacterial, therapy, and biosensing. Recently, the antimicrobial activities of rGO have been studied. Liu et al. [34] demonstrated the antibacterial activity against Escherichia coli with Gt, graphite oxide (GtO), GO, and reduced GO (rGO). Theirs results showed stronger antibacterial activity than GtO dispersion, and GO revealed the strongest antibacterial activity. The results also showed that antibacterial activities of Gt, GtO, GO, and rGO are concentration-dependent. Gurunathan et al. [35] assessed the P. aeruginosa growth curve with Gt, GtO, GO, and rGO. Their results proved the antibacterial activity of GO and rGO, which demonstrated that the antibacterial activities of graphene-based materials are reliant on the concentration. The antifungal activity was also studied where Sawangphruk et al. [36] showed that rGO totally inhibited the mycelia growth of Aspergillus niger, Aspergillus oryzae, and Fusarium oxysporum at concentrations 500, 500, and 250 µg/ml, respectively.
It has been known that silver nanoparticles (AgNPs) have antimicrobial properties and is widely used as highly efficient antimicrobial agent. In spite of this, AgNPs tends to agglomerate in the process of preparation [37, 38]. Several reports have been published on the rGO–Ag antimicrobial activity. For instant, Nguyen et al. [39] reported the antibacterial activity toward E. coli, Listonella anguillarum, Bacillus cereus, and S. aureus bacteria. Han et al. [37] investigated the antimicrobial activity against P. aeruginosa and suggested that RGO/AgNPs exhibited higher antimicrobial activity than AgNPs, owing to the synergic effect of RGO and AgNPs. Deng et al. [40] studied the synthesis of melamine sponge decorated with silver nanoparticles-modified graphene (G/AgNPs-MS) and investigated its antibacterial activity against E. coli and S. aureus bacteria. Fathalipour et al. [41] prepared l-cysteine modified AgNPs-decorated rGO nanocomposite and examined its antibacterial activity against E. coli and S. aureus. Bansala et al. [42] have recently studied the shielding properties of PVP-stabilized-AgNPs-based graphene nanohybrid. Minh Dat et al. [43] have synthesized silver/rGO (Ag/rGO) nanocomposites and have tested it against P. aeruginosa and S. aureus by optical density and plate colony-counting methods. Esmaeili et al. [44] have prepared nanofibrous scaffolds and in order to enhance its antimicrobial activity, rGO/Ag nanostructures were used into the mats. Ghosh et al. [45] fabricated rGO/Ag decorated conductive cotton fabric for high performing electromagnetic interference shielding and measured the zone of inhibition against E. coli.
To the best of our knowledge, the antimicrobial activity of FrGO and FrGO–Ag nanocomposite, in addition to its cytotoxicity toward dermal cells, has not been reported. This work aims to decrease the cytotoxicity of rGO through functionalization by PVP and to increase its antimicrobial properties by AgNPs decoration. The formulated FrGO–Ag nanocomposite with high antimicrobial activity and low cytotoxicity is meant to be an effective treatment for infected wound burns.
Results and discussion
UV–Vis spectroscopy
UV–Vis absorption spectrum [Fig. 1(A)(a)] of GO shows a characteristic peak at 231 nm and a shoulder at 300 nm, which correspond to π–π* transitions of C–C aromatic bonds and n–π* transitions of C=O bonds, respectively. These results agree with the previously reported data [12, 20, 21]. After reduction [Fig. 1(A)(b)], the appearance of a new absorption peak at 254 nm and the disappearance of 231 nm and 300 nm confirms the formation of rGO. These results are consistent with other authors [51]. Figure 1(A)(c) shows the absorption spectrum of FrGO, exhibiting complete disappearance of the characteristic band 231 nm and the appearance of a newly ill-defined band at 269 nm, suggesting the restoration of conjugated C=C bonds upon reduction [52].
X-ray diffraction
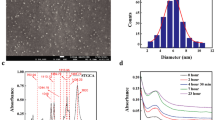
Figure 1B shows the XRD patterns of rGO (a) and FrGO (b). The pattern of rGO showed two characteristic peaks at 2θ = 23.37° and 42.99°, which are corresponding to d-value 3.8226 A and 2.1038 A, respectively [53]. The disappearance of peak at 11.07°, characteristic of GO sheets besides to the appearance of weak diffraction peak around 23° are evidence of successful reduction of GO sheets through removal of oxygen function groups on the surface of the sheets. Thus, the restacking of rGO sheets was easier to take place. Abulizi et al. [53] reported GO reduction to rGO through the disappearance of diffraction peak at 10.5 (002) and the formation of a new broad peak at around 24 (002) of rGO. While the peak appeared near 43° is due to presence of unexfoliated GtO. These results are in good agreement with reported results in the literature [21, 39, 54]. Figure 1(B)(b), two broad diffraction peaks appeared at 2θ values of about 14.41° and 21.77° which are assigned to the PVP. New peak also appeared at 2θ = 40.34° as a result of the functionalization of rGO by PVP.
XRD patterns in Fig. 1(c–e) confirmed the formation of Ag nanoparticles in the FrGO–Ag nanocomposites. FrGO–Ag nanocomposites prepared with different volumes of AgNO3 solutions showed four significant peaks at 2θ = 38.03°, 44.42°, 64.51°, and 77.49°. Those four peaks are corresponding (111), (200), (220), and (311) crystallographic planes of face-centered cubic (fcc) AgNPs, respectively (JCPDS card No. 07-0783). As seen in the figure, the intensity of AgNPs peaks increases by raising the volume of AgNO3 solution from 10 to 60 μl that indicates increasing in the degree of crystallinity of FrGO–Ag nanocomposite.
FTIR spectroscopy
Figure 2(A) represents the FTIR spectrum of: GO (a) and rGO (b) sheets, and Table 1 demonstrate the characteristic wavenumber (cm−1) of the main bands and their assignments. The FTIR spectra of GO sheets shows the characteristic peaks at 3368 cm−1, 1720 cm−1, 1621 cm−1, 1384 cm−1, 1250 cm−1, and 1067 cm−1 were due to OH stretching, C=O stretching, C=C stretching, OH deformation, epoxy C–O stretching, and alkoxy C–O stretching, respectively [55]. After reduction of GO sheets, the FTIR spectrum of rGO [Fig. 2(A)(b)] shows characteristic peaks at 3438 cm−1, 2923 cm−1, and 2854 cm−1 that correspond to OH stretching vibration [56, 57], CH2 symmetric, and asymmetric stretching vibrations, respectively. Moreover, a weak peak at 1722 cm−1 is corresponding to carbonyl (C=O) and a medium peak 1626 cm−1 assigned to carboxylic (C=C) stretching vibrations of the skeletal vibrations of graphitic backbone. In comparison with FTIR spectrum of GO, the intensity of carbonyl (C=O) stretching vibration at 1710 cm−1 decreased and the epoxy (C–O) stretching vibration at 1250 cm−1 disappeared, which confirm the removal of some of the oxygen functional groups of GO sheets. This result is in good agreement with recently reported in literature [58,59,60,61].
The FTIR spectrum of: FGO (a) and FrGO (b) sheets and FrGO–Ag nanocomposite (c) are illustrated in Fig. 2(B). FTIR spectra of FGO sheets [Fig. 2(B)(a)] shows broad peak at 3433 cm−1 which is ascribed to OH stretching vibration. A weak peak appeared at 1727 cm−1 and a strong peak at 1638 cm−1 which is ascribed to carbonyl (C=O) and carboxylic (C=C) stretching vibrations, respectively. Furthermore, the alkoxy C–O stretching group shifted from 1067 to 1038 cm−1. These findings confirm the removal of some of the oxygen functional groups of GO sheets. This result is in good agreement with recently reported studies [19, 21, 62].
Careful examination and comparing all the spectra revealed that FrGO sheets [Fig. 3(B)] exhibited noticeable decrease in the peak intensity of OH stretching vibration, carboxylic (C=C) stretching vibrations, epoxy (C–O) stretching, and alkoxy (C–O) stretching vibration at 3433 cm−1, 1636 cm−1, 1272 cm−1, and 1047 cm−1, respectively. These results indicate the loss of oxygen functional groups on FGO, which can be attributed to the successful reduction of FGO sheets.
Compared to FTIR spectrum of FrGO [Fig. 2(B)(b)], the carboxylic (C=C) stretching vibrations of FrGO–Ag nanocomposite are shifted to higher frequency from 1636 to 1644 cm−1. Another shift appeared to lower frequency in alkoxy (C–O) stretching vibration group from 1047 to 1037 cm−1. A strong peak at 1384 cm−1, which is assigned to OH deformation of C–OH, showed increase in intensity. This can be explained by attachment of AgNPs to FrGO sheets. On the other side, the peaks at 1725 cm−1, 1644 cm−1, 1285 cm−1, and 1037 cm−1 revealed decrease in intensity. This may be contributed to the reduction of AgNO3 by using NaBH4 which reduces carbonyl and epoxy groups causes residual hydroxyl function groups. Yang and colleagues [63] reported that aldehydes and ketones have been frequently reduced to alcohol by NaBH4.
Raman spectroscopy
Figure 3(A) (a, b) shows Raman spectra of GO (a), and rGO (b), respectively. The spectrum of rGO sheets displays much more intense and resolved D and G bands at 1352 cm−1 and 1596 cm−1 compared with those of the GO sheets. The G band (Fig. 3) of rGO sheets shifted from lower to higher frequency (from 1592 to 1596 cm−1). The intensity ratio of D to G band (ID/IG) increased from 0.87 (GO) to 1.05 (rGO), which confirms the reduction of GO to rGO sheets. The result is in good agreement with Hareesh et al. [64].
The D band and G band of FrGO sheets [Fig. 3(B)(b)] shifted to lower frequency from 1356 to 1348 cm−1 and from 1604 to 1592 cm−1, respectively due to the reduction of FGO sheets. The intensity ratio of both D and G band (ID/IG) of FrGO sheets increased from 0.86 to 0.96, which is attributed to the reduction of FGO sheets [Fig. 3(A)(b)]. The intensity ratio of D to G band (ID/IG) of FrGO sheets increased from 0.86 to 0.96, which is attributed to the reduction of FGO sheets. These Raman results agree well with those reported by Yoon and In [65]. Stankovich et al. [66] attributed the increase of the intensity ratio (ID/IG) in Raman spectra of rGO sheets to the decrease in the average size of sp2 domains due to reduction of the GO. Weng et al. [20] stated that the ID/IG is considered as an indicator to evaluate the size of sp2 domains and the structural disorder of the graphene nanosheet.
After decoration of FrGO by AgNPs, the D band and G band of FrGO–Ag nanocomposite [Fig. 3(B)(c)] shifted to lower frequency from 1348 to 1344 cm−1 and from 1591 to 1584 cm−1, respectively. In addition, the ID/IG of FrGO–Ag nanocomposite decreased from 0.96 to 0.93 upon the decoration of AgNPs. This result agrees with the reported by Han et al. [37]. It could be observed that an apparent enhancement in Raman signals after the decoration with the AgNPs owing to the plasmon effect of Ag nanoparticles, which is in consistent with Huang et al. [67].
HR-TEM
Figure 4(a) representing the HRTEM micrographs of rGO sheets, and FrGO sheets, displayed in Fig. 4(b) show surface modification by PVP. The HRTEM micrographs of FrGO–Ag nanocomposites prepared with different amounts of AgNO3 solution (10, 30, and 60 μl) for a constant amount of NaBH4 solution are illustrated in Fig. 4(c–e), respectively. The micrographs represent Ag nanoparticles with small particle sizes, which are freely embedded between FrGO sheets. Figure 4(c) shows a small amount of Ag nanoparticles, while Fig. 4(d, e) show a larger amount of Ag nanoparticles. This owes to the amount of AgNO3 solution (60 µl) used in the preparation. In addition to some agglomerations of Ag nanoparticles that appeared clearly in Fig. 4(e).
Antibacterial activity
Contact time effect
The antimicrobial activity of rGO, FrGO sheets, FrGO–Ag nanocomposites of fixed concentration (100 µg/ml) is illustrated in Fig. 5(a–c) for different periods of time up to two hours against S. aureus, P. aeruginosa, and C. albicans, respectively.
The rGO sheets showed a sharp decrease in the colony count up to 60 min (around 65%) and reached about 70% after 120 min. This result is found to be in good agreement with those reported in literature. For instance, Gurunathan et al. [35] treated P. aeruginosa with rGO with a constant concentration (75 µg/ml) and found that rGO had a negative effect on bacterial growth, as the turbidity measurements decreased steadily over a 6 h period. After 15 h of exposure to GO, and rGO, there was significant growth inhibition of up to 92%. Akhavan and Ghaderi [68] reported the antibacterial activity of rGO nanowalls (rGONWs) toward E. coli and S. aureus. They found that rGONW exhibited stronger antibacterial activity than their activities against E. coli bacteria. Actually after 1 h, only 5(± 1) % of the S. aureus bacteria was observed on the surface of RGNWs. Although, our results agree with the above-mentioned authors, they disagree with the results reported by Min Peng et al. [69], who reported no significant antimicrobial activity of rGO against oral pathogens namely; C. albicans, Lactobacillus acidophilus, Streptococcus mutans.
The FrGO sheets showed slight antimicrobial activity (around 9%) for almost all the microorganisms. This could be attributed to the functionalization of rGO by PVP, which hindered the oxygen functional group found on the surface of rGO sheets. After decoration of FrGO with AgNPs, apparent inhibition of the colony count occurred, especially for FrGO–Ag nanocomposite which was prepared with high amounts (50 and 60 µl/ml) of AgNO3. In Particular, FrGO–Ag nanocomposite prepared with low amounts (10, 20, 30, and 40 µl/ml) of AgNO3, showed a gradual decline (range of 58–78%) in the colony count up to 90 min and reach total inhibition after 120 min. On the other hand, FrGO–Ag nanocomposite prepared with high amounts (50 and 60 µl/ml) of AgNO3, achieved a maximum loss in colony count of 69% after 60 min and reached total colony loss after 90 min. To sum up, the antimicrobial activity has been enhanced in FrGO–Ag nanocomposite which has been prepared with 60 μl/ml of AgNO3 due to the decoration FrGO sheets by AgNPs for P. aeruginosa, S. aureus, and C. albicans by nearly 100%, 96%, and 90.5%, respectively.
Dose effect
The minimum dose of inhibition was investigated by using the colony count method for 2 h incubation. The antimicrobial activity of different doses of (25, 50, 75, 100 µg/ml) of rGO, FrGO sheets, and FrGO–Ag nanocomposites (prepared by different amounts of 10, 20, 30, 40, 50, and 60 µl/ml of AgNO3) against S. aureus, P. aeruginosa, and C. albicans was studied and illustrated in Fig. 6(a–c), respectively. Despite the fact that rGO sheets at a concentration of 100 g/ml had a significant effect on colony counts, with a maximum percentage of microorganism killed of approximately 77%, FrGO sheets at the same concentration had only a minor antimicrobial effect, with nearly 8% colony loss. The antibacterial properties of rGO against P. aeruginosa are consistent with the findings of Gurunathan et al. [35] who demonstrated antibacterial properties of rGO against P. aeruginosa incubated for 2 h at 37 °C. They demonstrated that when the cells were exposed to rGO, significant loss was observed at concentrations of 75 µg/ml and 100 µg/ml and maximum loss of P. aeruginosa viability was detected after incubation with 150 µg/ml of rGO.
For both Gram positive (S. aureus) and gram negative (P. aeruginosa) bacteria, the antibacterial activity of FrGO–Ag nanocomposite showed similar behavior. However, FrGO–Ag nanocomposites prepared at low concentrations (10, 20, and 30 µl/ml) of AgNO3 showed total loss at the dose of 100 µg/ml, and those prepared at high concentrations (40, 50, and 60 µl/ml) of AgNO3 showed total loss at the dose of 75 µg/ml.
Upon treating the C. albicans 100% elimination of colony count is observed at the dose of 75 µg/ml for FrGO–Ag nanocomposite (prepared with high AgNO3 amounts of 50 and 60 µl/ml), and at the dose of 100 µg/ml FrGO–Ag nanocomposite (prepared with low AgNO3 amounts of 10, 20, 30, and 40 µl/ml). FrGO–Ag nanocomposite (75 µg/ml) prepared with 10 and 20 g/ml AgNO3 reduced colony counts by approximately 51 and 61%, respectively. Whereas, FrGO–Ag nanocomposite (75 µg/ml) which was prepared with amounts of 30 and 40 µl/ml of AgNO3 exhibited nearly a 69% and a 72% reduction in colony count, respectively.
It could be concluded that decoration of FrGO with AgNPs prepared using high AgNO3 amounts (50 and 60 µl/ml) significantly improves its antimicrobial activity nearly by 92%, leading to a total loss in colony counts of all studied microorganisms at the dose of 75 μg/ml.
Antimicrobial mechanism
The untreated bacterial (S. aureus) cell and the FrGO–Ag adhered to the treated bacterial (S. aureus) cell are shown in Fig. 7. As shown in Fig. 7(a), untreated S. aureus bacteria show a well-integrated cell wall. Following direct contact with FrGO–Ag, the bacterial cell wall [Fig. 7(b–d)] appeared to loss its integrity and damage into pieces, followed by the total cellular damage of S. aureus. This irreversible damage could be explained by the direct contact of FrGO–Ag with the bacterial cell, assisting the physical damage through sharp edges. Meanwhile, the AgNPs released into the cytoplasm caused cellular disruption. It could be concluded that the antibacterial mechanism of functionalized graphene-based materials decorated with AgNPs based on the synergistic effect of graphene-based materials and AgNPs.
Cytotoxicity
Figure 8 shows the cytotoxic behavior of rGO, FrGO, and FrGO–Ag nanocomposite. After 48 h of incubation with the materials under investigation, cytotoxicity was assessed against the BJ1 normal human epithelial cell line at concentrations ranging from 100 to 0.78 μg/ml. The reduction of GO enhanced its cell viability effect by 10%, where about 35.8% of the cells are viable at rGO sheets against 25.8% at GO. In comparison with the cytotoxicity of FGO, the cell viability of FrGO was reduced (~ 12%) after a reduction from 64.4 to 52.5%. It could be concluded that functionalization of rGO improved the cell viability from 35.8 to 52.5% and decoration of FrGO sheets by AgNPs enhanced the cell viability from 52.5 to 88%.
Conclusion
It could be concluded from the results that rGO, and FrGO are dose- and time-dependent. The antimicrobial activity of FrGO–Ag nanocomposite prepared with 60 μl/ml of AgNO3 has been increased owing to the decoration of FrGO sheets by AgNPs, which is nearly 100%, 96%, and 90.5% for P. aeruginosa, S. aureus, and C. albicans, respectively. The reduction of GO sheets reduced the cytotoxicity by about 10%, and the functionalization of rGO sheets by PVP reduced the cytotoxicity by nearly 16.7%. Finally, the biocompatibility of FrGO–Ag nanocomposite against the BJ1 normal human epithelial cell line reached up to 88% cell viability.
Materials and methods
Materials
In this work, Synthetic graphite Gt powder was purchased from Sigma-Aldrich, USA, with a particle size of < 20 µm. Potassium permanganate KMnO4 (99.0%) was supplied by Laboratory Rasayan, Gujarat, India. Sulfuric acid S.G. H2SO4 (> 95%) and PVP (molecular biology grade) (C6H9NO)n with average F.M. 40,000 (g/mol) were purchased from Fisher, USA. Hydrogen peroxide H2O2 (30%) was bought from Carl Roth, Karlsruhe, Germany. Sodium nitrate NaNO3 (99%) was from Acros, Geel, Antwerp Belgium and l-ascorbic acid l-A.A. (M = 176.13 g/mol) ACS was from Fisher, USA. Sodium borohydride fine granular NaBH4 (98%) was purchased from (Russian Federation) Merck and Silver nitrate AgNO3 extra pure was from Sisco Research Laboratories (SRL), E, Mumbai, Maharashtra 400099, India. Milli-Q water was used during the sample preparation. All the purchased chemicals used without further purification.
Methods
Preparation of graphene oxide (GO) sheets
According to the modified Hummers and Offeman’s method, GO was prepared from the Gt powder as reported in our previous work [46, 47]. NaNO3 (5 g) was added to H2SO4 (230 ml) in an ice bath, followed by 10 g of Gt (10 g) powder. KMnO4 was added slowly under violent stirring. Drop by drop, 450 ml of distilled water was added into the flask where the temperature was controlled. Then, 1400 ml of hot distilled water was added under stirring. The reaction was completed by cautious and gentle addition of 50 ml of H2O2. The resulting Gt was filtered and repeatedly rinsed with dist. water till its pH reached 4.5. The washing process was achieved by centrifugation and dialysis to eliminate the residual acids, metal ions and acids. By ultrasonication of Gt sheets for 30 min, the exfoliated GO sheets were obtained and they were dried at 50 °C.
Preparation of reduced graphene oxide (rGO) sheets
GO solution of concentration (0.01 mg/ml) was dispersed by ultrasound irradiation for 30 min continuously in an ice bath to avoid thermal stress. Ultrasound irradiation was performed by Hummer sonicator. 0.138 g of l-A.A. was added to the dispersed GO under stirring. A few drops of NaOH (1 M) were used to elevate the pH of the medium up to pH 11. The obtained solution was sonicated by performing a pulse mode of 50 s on/50 s off. Sonication occurred at 60 °C for 2 h till the solution turned from a brown color to a brown black color. The obtained sample was filtered immediately and washed several times using de-ionized water and absolute ethanol to get rid of the excess l-A.A, finally dried at 50 °C.
Preparation of functionalized graphene oxide (FGO) sheets
Using a hummer sonicator, the GO solution of concentration (0.25 mg/ml) was dispersed for 30 min continuously. To control the increase in temperature, the GO solution was dispersed in ice sonicator. Then, PVP (0.25 g) and l-A.A. (0.5 g) were added. The resulted mixture was incubated at 80 °C for 4 h. Immediately after the incubation, the obtained solution was filtered by sterilized cellulose nitrate membrane filter. The excess dissolved PVP and l-A.A. was removed by repeated washing then dried at 50 °C.
Preparation of functionalized reduced graphene oxide (FrGO) sheets
Dry FGO was dispersed in 1 ml de-ionized water (0.25 mg/ml) for 1 h (pulsed mode: 50 s on/10 s off) by hummer sonicator. 20 µl of NaBH4 solution (0.1 M) was added under stirring. After 15 min of stirring, the whole solution was filtered and washed with de-ionized water. The FrGO sheets were obtained by drying the sample at 50 °C.
Preparation of functionalized reduced graphene oxide–silver (FrGO–Ag) nanocomposites
A solution of dispersed FGO (0.05 mg/ml) was prepared and dispersed under a hummer sonicator for 1 h (pulsed mode: 50 s on/10 s off) in an ice bath. Under stirring, 10 µl of AgNO3 solution was added to the dispersed solution of FGO, and then 20 µl of NaBH4 (0.1 M) was added to the mixture. With continuous stirring for 15 min, the sample turned from pale black to a yellowish black color. The obtained sample was filtered by sterilized cellulose nitrate membrane filter of 0.45 µ pore size and washed 3 times with de-ionized water. To obtain the functionalized reduced graphene silver (FrGO–Ag) nanocomposite, the obtained sample was dried at 50 °C.
A series of functionalized reduced graphene silver nanocomposites was prepared with variable volumes of AgNO3 solution (20, 30, 40, 50, and 60 µl) and NaBH4 solution (20, 40, 60, 80, 100, and 120 µl). The obtained solution was filtered and repeatedly washed 3 times by de-ionized water. Then obtained sample was dried at 50 °C.
Antibacterial activity
Microorganisms used and preparation
Staphylococcus aureus (ATCC6538), P. aeruginosa (ATCC15442), and C. albicans (ATCC10231) were grown in tryptic soya broth (purchased from Thermo Fisher Scientific Oxoid-UK) at 37 °C during 24 h, their suspensions were homogenized and centrifuged at 3000 rpm for 20 min. The sterile phosphate buffered solution was used in washing three times. The density of each strain inocula was 106 CFU/ml.
Determination of antimicrobial effect
The antimicrobial effect and minimum inhibitory concentration (MIC) of prepared materials were determined against the above-mentioned microbial strains [47]. Each one of stock suspension previously prepared was exposed to four concentrations of the materials under investigation (25, 50, 75, and 100 µg/ml) at three contact times (60, 90, and 120 min).
MTT Cytotoxicity test
The cytotoxicity of composites was reported in our previous work [48]. In brief, for non-radioactive quantification of cell proliferation and cell viability, the cytotoxicity of composites was determined by cell proliferation kit 1 (MTT assay). CO2 incubator (Sheldon, TC2323, Cornelius, OR, USA) was used to incubate the normal skin fibroblast cell in humidified 5% CO2 at 37 °C. Various concentrations (from 100 to 0.78 µg/ml) of the composite were added to the cells which were aliquoted in 96-well microliter plastic plates. The cells were then incubated for 48 h. Then, 200 µl of sodium dodecyl sulfate was added to stop the reaction and incubated 12 h at 37 °C. The microplate reader (Bio-Rad Laboratories, model 3350, USA) was used to measure the formazan amount produced at wavelength 595 nm.
Characterization
The UV–Vis absorption spectra were measured in the range between 200 and 800 nm using JASCO V-630 spectrophotometer, Japan. Fourier transform infrared (FTIR) spectra of the prepared samples was recorded by Bruker-VERTEX 70, Germany. The high-resolution transmission electron microscope HRTEM (JEOL Model, JEM-2100, Japan) was used to study the morphological structures of rGO, FrGO, and FrGO–Ag nanocomposite. X-ray diffraction (XRD) patterns were obtained by XRD (X’Pert Pro PANalytical, Holland), targeted by Cu Kα with secondary monochromator (45 kV, 40 mA).
Data availability
All data generated or analyzed during this study are included in this published article.
References
V. Singh, D. Joung, L. Zhai, S. Das, S.I. Khondaker, S. Seal, Graphene based materials: past, present and future. Prog. Mater. Sci. 56, 1178–1271 (2011). https://doi.org/10.1016/j.pmatsci.2011.03.003
J. An, Y. Gou, C. Yang, F. Hu, C. Wang, Synthesis of a biocompatible gelatin functionalized graphene nanosheets and its application for drug delivery. Mater. Sci. Eng. C 33, 2827–2837 (2013). https://doi.org/10.1016/j.msec.2013.03.008
M. Rasheed, S. Shihab, O.W. Sabah, An investigation of the structural, electrical and optical properties of graphene-oxide thin films using different solvents. J. Phys. Conf. Ser. (2021). https://doi.org/10.1088/1742-6596/1795/1/012052
P.A.A.P. Marques, G. Gonçalves, S. Cruz, N. Almeida, M.K. Singh, J. Grácio, A.C.M. Sousa, Functionalized graphene nanocomposites. In Advances in Nanocomposite Technology (InTech Open, London, 2011), pp. 248–272. https://doi.org/10.5772/18209
A.K. Geim, K.S. Novoselov, The rise of graphene. Nat. Mater. 6, 183–191 (2007). https://doi.org/10.1038/nmat1849
K.I. Bolotin, K.J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim, H.L. Stormer, Ultrahigh electron mobility in suspended graphene. Solid State Commun. 146, 351–355 (2008). https://doi.org/10.1016/j.ssc.2008.02.024
C. Lee, X. Wei, J.W. Kysar, J. Hone, Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science (1979) 321, 385–388 (2008). https://doi.org/10.1126/science.1157996
Y. Jiang, Q. Zhang, F. Li, L. Niu, Glucose oxidase and graphene bionanocomposite bridged by ionic liquid unit for glucose biosensing application. Sens. Actuators B 161, 728–733 (2012). https://doi.org/10.1016/j.snb.2011.11.023
C.V. Pham, M. Eck, M. Krueger, Thiol functionalized reduced graphene oxide as a base material for novel graphene–nanoparticle hybrid composites. Chem. Eng. J. 231, 146–154 (2013). https://doi.org/10.1016/j.cej.2013.07.007
M.D. Stoller, S. Park, Z. Yanwu, J. An, R.S. Ruoff, Graphene-based ultracapacitors. Nano Lett. 8, 3498–3502 (2008). https://doi.org/10.1021/nl802558y
A.A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao, C.N. Lau, Superior thermal conductivity of single-layer graphene. Nano Lett. 8, 902–907 (2008). https://doi.org/10.1021/nl0731872
Y. Zhu, S. Murali, W. Cai, X. Li, J.W. Suk, J.R. Potts, R.S. Ruoff, Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924 (2010). https://doi.org/10.1002/adma.201001068
S. Deng, J. Lei, L. Cheng, Y. Zhang, H. Ju, Amplified electrochemiluminescence of quantum dots by electrochemically reduced graphene oxide for nanobiosensing of acetylcholine. Biosens. Bioelectron. 26, 4552–4558 (2011). https://doi.org/10.1016/j.bios.2011.05.023
T. Kuila, S. Bose, A.K. Mishra, P. Khanra, N.H. Kim, J.H. Lee, Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 57, 1061–1105 (2012). https://doi.org/10.1016/j.pmatsci.2012.03.002
Y. Tian, Z. Yu, L. Cao, X.L. Zhang, C. Sun, D.W. Wang, Graphene oxide: an emerging electromaterial for energy storage and conversion. J. Energy Chem. 55, 323–344 (2021). https://doi.org/10.1016/j.jechem.2020.07.006
A.S. Wajid, S. Das, F. Irin, H.S.T. Ahmed, J.L. Shelburne, D. Parviz, R.J. Fullerton, A.F. Jankowski, R.C. Hedden, M.J. Green, Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production. Carbon (NY) 50, 526–534 (2011). https://doi.org/10.1016/j.carbon.2011.09.008
S. Yoon, I. In, Role of poly(N-vinyl-2-pyrrolidone) as stabilizer for dispersion of graphene via hydrophobic interaction. J. Mater. Sci. 46, 1316–1321 (2011). https://doi.org/10.1007/s10853-010-4917-2
M. Contardi, D. Kossyvaki, P. Picone, M. Summa, X. Guo, J.A. Heredia-Guerrero, D. Giacomazza, R. Carzino, L. Goldoni, G. Scoponi, F. Rancan, R. Bertorelli, M. Di Carlo, A. Athanassiou, I.S. Bayer, Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. (2021). https://doi.org/10.1016/j.cej.2020.128144
D. Han, C. Shan, L. Guo, L. Niu, D. Han, Electro-oxidation of ascorbic acid on PVP-stabilized graphene electrode. Chem. Res. Chin. Univ. 26, 287–290 (2010)
X. Weng, B. Li, Y. Zhang, X. Lv, G. Gu, Synthesis of flake shaped carbonyl iron/reduced graphene oxide/polyvinyl pyrrolidone ternary nanocomposites and their microwave absorbing properties. J. Alloys Compd. 695, 508–519 (2017). https://doi.org/10.1016/j.jallcom.2016.11.083
Y. Zhang, H. Chi, W. Zhang, Y. Sun, Q. Liang, Y. Gu, R. Jing, Highly efficient adsorption of copper ions by a PVP-reduced graphene oxide based on a new adsorptions mechanism. NANO–MICRO Lett. 6, 80–87 (2014)
B. Yin, J. Wang, H. Jia, J. He, X. Zhang, Z. Xu, Enhanced mechanical properties and thermal conductivity of styrene–butadiene rubber reinforced with polyvinylpyrrolidone-modified graphene oxide. J. Mater. Sci. 51, 5724–5737 (2016). https://doi.org/10.1007/s10853-016-9874-y
Z. Zhang, W. Zhang, D. Li, Y. Sun, Z. Wang, C. Hou, L. Chen, Y. Cao, Y. Liu, Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in situ method. Int. J. Mol. Sci. 16, 2239–2251 (2015). https://doi.org/10.3390/ijms16012239
M. Contardi, D. Kossyvaki, P. Picone, M. Summa, X. Guo, J.A. Heredia-Guerrero, D. Giacomazza, R. Carzino, L. Goldonic, G. Scoponi, F. Rancan, R. Bertorelli, M. di Carlo, A. Athanassiou, I.S. Bayer, Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 409, 128144 (2021). https://doi.org/10.1016/j.cej.2020.128144
M. Contardi, D. Russo, G. Suarato, J.A. Heredia-Guerrero, L. Ceseracciu, I. Penna, N. Margaroli, M. Summa, R. Spanò, G. Tassistro, L. Vezzulli, T. Bandiera, R. Bertorelli, A. Athanassiou, I.S. Bayer, Polyvinylpyrrolidone/hyaluronic acid-based bilayer constructs for sequential delivery of cutaneous antiseptic and antibiotic. Chem. Eng. J. 358, 912–923 (2019). https://doi.org/10.1016/j.cej.2018.10.048
U.C. Paul, G. Bayer, S. Grasselli, A. Malchiodi, I.S. Bayer, Biodegradable, stretchable and transparent plastic films from modified waterborne polyurethane dispersions. Polymers (Basel) 14, 1199 (2022). https://doi.org/10.3390/polym14061199
I. Lee, S.M. Kang, S.C. Jang, G.W. Lee, H.E. Shim, M. Rethinasabapathy, C. Roh, Y.S. Huh, One-pot gamma ray-induced green synthesis of a Prussian blue-laden polyvinylpyrrolidone/reduced graphene oxide aerogel for the removal of hazardous pollutants. J. Mater. Chem. A 7, 1737–1748 (2019). https://doi.org/10.1039/c8ta10250c
A. Pavinatto, L.A. Mercante, M.H.M. Facure, R.B. Pena, R.C. Sanfelice, L.H.C. Mattoso, D.S. Correa, Ultrasensitive biosensor based on polyvinylpyrrolidone/chitosan/reduced graphene oxide electrospun nanofibers for 17α-ethinylestradiol electrochemical detection. Appl. Surf. Sci. 458, 431–437 (2018). https://doi.org/10.1016/j.apsusc.2018.07.035
C. Huang, Y. Ding, C. Hao, S. Zhou, X. Wang, H. Gao, L. Zhu, J. Wu, PVP-assisted growth of Ni–Co oxide on N-doped reduced graphene oxide with enhanced pseudocapacitive behavior. Chem. Eng. J. 378, 122202 (2019). https://doi.org/10.1016/j.cej.2019.122202
B. Li, X. Weng, X. Sun, Y. Zhang, X. Lv, G. Gu, Facile synthesis of Fe3O4/reduced graphene oxide/polyvinyl pyrrolidone ternary composites and their enhanced microwave absorbing properties. J. Saudi Chem. Soc. 22, 979–984 (2018). https://doi.org/10.1016/j.jscs.2018.02.006
Y. Gao, X. Zou, J.X. Zhao, Y. Li, X. Su, Graphene oxide-based magnetic fluorescent hybrids for drug delivery and cellular imaging. Colloids Surf. B 112, 128–133 (2013). https://doi.org/10.1016/j.colsurfb.2013.07.020
B.S. Dash, G. Jose, Y.J. Lu, J.P. Chen, Functionalized reduced graphene oxide as a versatile tool for cancer therapy. Int. J. Mol. Sci. 22, 1–24 (2021). https://doi.org/10.3390/ijms22062989
C. Shan, H. Yang, D. Han, Q. Zhang, A. Ivaska, L. Niu, Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosens. Bioelectron. 25, 1504–1508 (2010). https://doi.org/10.1016/j.bios.2009.11.009
S. Liu, T.H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5(9), 6971–6980 (2011). https://doi.org/10.1021/nn202451x
S. Gurunathan, J.W. Han, A.A. Dayem, V. Eppakayala, J.H. Kim, Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 7, 5901–5914 (2012). https://doi.org/10.2147/IJN.S37397
M. Sawangphruk, P. Srimuk, P. Chiochan, T. Sangsri, P. Siwayaprahm, Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon (NY) 50, 5156–5161 (2012). https://doi.org/10.1016/j.carbon.2012.06.056
Y. Han, Z. Luo, L. Yuwen, J. Tian, X. Zhu, L. Wang, Synthesis of silver nanoparticles on reduced graphene oxide under microwave irradiation with starch as an ideal reductant and stabilizer. Appl. Surf. Sci. 266, 188–193 (2013). https://doi.org/10.1016/j.apsusc.2012.11.132
C. Lok, C. Ho, R. Chen, Q. He, W. Yu, H. Sun, P.K. Tam, J. Chiu, C. Che, Proteomic analysis of the mode of antibacterial action of silver nanoparticles research articles. J. Proteome Res. 5, 916–924 (2006)
V.H. Nguyen, B.K. Kim, Y.L. Jo, J.J. Shim, Preparation and antibacterial activity of silver nanoparticles-decorated graphene composites. J. Supercrit. Fluids 72, 28–35 (2012). https://doi.org/10.1016/j.supflu.2012.08.005
C.-H. Deng, J.-L. Gong, P. Zhang, G.-M. Zeng, B. Song, H.-Y. Liu, Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 488, 26–38 (2017). https://doi.org/10.1016/j.jcis.2016.10.078
S. Fathalipour, S. Pourbeyram, A. Sharafian, A. Tanomand, P. Azam, Biomolecule-assisted synthesis of Ag/reduced graphene oxide nanocomposite with excellent electrocatalytic and antibacterial performance. Mater. Sci. Eng. C 75, 742–751 (2017). https://doi.org/10.1016/j.msec.2017.02.122
T. Bansala, S. Mukhopadhyay, M. Joshi, R. Doong, M. Chaudhary, Synthesis and shielding properties of PVP-stabilized-AgNPs-based graphene nanohybrid in the Ku band. Synth. Met. 221, 86–94 (2016). https://doi.org/10.1016/j.synthmet.2016.07.034
N. Minh Dat, V.N.P. Linh, L.A. Huy, N.T. Huong, T.H. Tu, N.T.L. Phuong, H.M. Nam, M. Thanh Phong, N.H. Hieu, Fabrication and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus of silver nanoparticle decorated reduced graphene oxide nanocomposites. Mater. Technol. 34, 369–375 (2019). https://doi.org/10.1080/10667857.2019.1575555
E. Esmaeili, T. Eslami-Arshaghi, S. Hosseinzadeh, E. Elahirad, Z. Jamalpoor, S. Hatamie, M. Soleimani, The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 152, 418–427 (2020). https://doi.org/10.1016/j.ijbiomac.2020.02.295
S. Ghosh, S. Ganguly, P. Das, T.K. Das, M. Bose, N.K. Singha, A.K. Das, N.C. Das, Fabrication of reduced graphene oxide/silver nanoparticles decorated conductive cotton fabric for high performing electromagnetic interference shielding and antibacterial application. Fibers Polym. 20, 1161–1171 (2019). https://doi.org/10.1007/s12221-019-1001-7
M.A.K. Moharram, K. Tohami, W.M. El Hotaby, A.M. Bakr, Graphene oxide porous crosslinked cellulose nanocomposite microspheres for lead removal: kinetic study. React. Funct. Polym. (2016). https://doi.org/10.1016/j.reactfunctpolym.2016.02.001
W.A. Khalil, H.H.A. Sherif, B.A. Hemdan, S.K.H. Khalil, W. El Hotaby, Biocompatibility enhancement of graphene oxide–silver nanocomposite by functionalisation with polyvinylpyrrolidone. IET Nanobiotechnol. (2019). https://doi.org/10.1049/iet-nbt.2018.5321
W. El Hotaby, H.H.A. Sherif, B.A. Hemdan, W.A. Khalil, S.K.H. Khalil, Assessment of in situ-prepared polyvinylpyrrolidone–silver nanocomposite for antimicrobial applications. Acta Phys. Pol. A (2017). https://doi.org/10.12693/APhysPolA.131.1554
A.F. De Faria, D.S.T. Martinez, S.M.M. Meira, A.C.M. de Moraes, A. Brandelli, A.G.S. Filho, O.L. Alves, Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B 113, 115–124 (2014). https://doi.org/10.1016/j.colsurfb.2013.08.006
X. Zhi, H. Fang, C. Bao, G. Shen, J. Zhang, K. Wang, S. Guo, T. Wan, D. Cui, The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials 34, 5254–5261 (2013). https://doi.org/10.1016/j.biomaterials.2013.03.024
M. Zainy, N.M. Huang, S. Vijay Kumar, H.N. Lim, C.H. Chia, I. Harrison, Simple and scalable preparation of reduced graphene oxide–silver nanocomposites via rapid thermal treatment. Mater. Lett. 89, 180–183 (2012). https://doi.org/10.1016/j.matlet.2012.08.101
X. Zhang, J. Wang, H. Jia, S. You, X. Xiong, L. Ding, Z. Xu, Multifunctional nanocomposites between natural rubber and polyvinyl pyrrolidone modified graphene. Composites B 84, 121–129 (2016). https://doi.org/10.1016/j.compositesb.2015.08.077
A. Abulizi, K. Okitsu, J.J. Zhu, Ultrasound assisted reduction of graphene oxide to graphene in l-ascorbic acid aqueous solutions: kinetics and effects of various factors on the rate of graphene formation. Ultrason. Sonochem. 21, 1174–1181 (2014). https://doi.org/10.1016/j.ultsonch.2013.10.019
Y. Yao, S. Miao, S. Liu, L. Ping, H. Sun, S. Wang, Synthesis, characterization, and adsorption properties of magnetic Fe3O4@ graphene nanocomposite. Chem. Eng. J. 184, 326–332 (2012). https://doi.org/10.1016/j.cej.2011.12.017
C. Bora, P. Bharali, S. Baglari, S.K. Dolui, B.K. Konwar, Strong and conductive reduced graphene oxide/polyester resin composite films with improved mechanical strength, thermal stability and its antibacterial activity. Compos. Sci. Technol. 87, 1–7 (2013). https://doi.org/10.1016/j.compscitech.2013.07.025
P.J. Wang, D. Zhou, H.H. Guo, W.F. Liu, J.Z. Su, M. Sen Fu, C. Singh, S. Trukhanov, A. Trukhanov, Ultrahigh enhancement rate of the energy density of flexible polymer nanocomposites using core–shell BaTiO3@MgO structures as the filler. J. Mater. Chem. A (2020). https://doi.org/10.1039/d0ta03304a
M. Yosef, A. Fahmy, W. El Hotaby, A.M. Hassan, A.S.G. Khalil, B. Anis, High performance graphene-based PVF foam for lead removal from water. J. Mater. Res. Technol. (2020). https://doi.org/10.1016/j.jmrt.2020.08.011
J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang, S. Guo, Reduction of graphene oxide via l-ascorbic acid. Chem. Commun. 46, 1112–1114 (2010). https://doi.org/10.1039/b917705a
Z. Yang, Q. Zheng, H. Qiu, J. Li, J. Yang, A simple method for the reduction of graphene oxide by sodium borohydride with CaCl2 as a catalyst. N. Carbon Mater. 30, 41–47 (2015). https://doi.org/10.1016/S1872-5805(15)60174-3
G. Neri, S.G. Leonardi, M. Latino, N. Donato, S. Baek, D.E. Conte, P.A. Russo, N. Pinna, Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens. Actuators B 179, 61–68 (2013). https://doi.org/10.1016/j.snb.2012.10.031
V.H. Nguyen, C. Lamiel, D. Kharismadewi, V.C. Tran, J.J. Shim, Covalently bonded reduced graphene oxide/polyaniline composite for electrochemical sensors and capacitors. J. Electroanal. Chem. 758, 148–155 (2015). https://doi.org/10.1016/j.jelechem.2015.10.023
A. Moayeri, A. Ajji, High capacitance carbon nanofibers from poly(acrylonitrile) and poly(vinylpyrrolidone)-functionalized graphene by electrospinning. J. Nanosci. Nanotechnol. 17, 1820–1829 (2017). https://doi.org/10.1166/jnn.2017.12877
L. Wang, R. Yang, H. Wang, J. Li, L. Qu, P.B. de Harrington, High-selective and sensitive voltammetric sensor for butylated hydroxyanisole based on AuNPs–PVP–graphene nanocomposites. Talanta 138, 169–175 (2015). https://doi.org/10.1016/j.talanta.2015.01.016
K. Hareesh, R.P. Joshi, S.S. Dahiwale, V.N. Bhoraskar, S.D. Dhole, Synthesis of Ag-reduced graphene oxide nanocomposite by gamma radiation assisted method and its photocatalytic activity. Vacuum 124, 40–45 (2016). https://doi.org/10.1016/j.vacuum.2015.11.011
S. Yoon, I. In, Role of poly (N-vinyl-2-pyrrolidone) as stabilizer for dispersion of graphene via hydrophobic interaction. J. Mater. Sci. 46, 1316–1321 (2011). https://doi.org/10.1007/s10853-010-4917-2
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T.B.T. Nguyen, R.S. Ruoff, Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon (NY) 45, 1558–1565 (2007). https://doi.org/10.1016/j.carbon.2007.02.034
Q. Huang, J. Wang, W. Wei, Q. Yan, C. Wu, X. Zhu, A facile and green method for synthesis of reduced graphene oxide/Ag hybrids as efficient surface enhanced Raman scattering platforms. J. Hazard. Mater. 283, 123–130 (2014). https://doi.org/10.1016/j.jhazmat.2014.09.021
O. Akhavan, E. Ghaderi, Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4, 5731–5736 (2010). https://doi.org/10.1021/nn101390x
J. Min Peng, J. Cheng Lin, Z. Yu Chen, M. Chao Wei, Y. Xiang Fu, S. Shen Lu, D. Sheng Yu, W. Zhao, Enhanced antimicrobial activities of silver-nanoparticle-decorated reduced graphene nanocomposites against oral pathogens. Mater. Sci. Eng. C 71, 10–16 (2017). https://doi.org/10.1016/j.msec.2016.09.070
Acknowledgments
This work was funded and supported by Science and Technology Development Fund STDF (Fund Number 12645).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research carried out within this work did not involve human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sherif, H.H.A., El Hotaby, W., Khalil, S.K.H. et al. Preparation, characterization, and biological assessment of functionalized reduced graphene oxide–silver nanocomposite. Journal of Materials Research 38, 1843–1857 (2023). https://doi.org/10.1557/s43578-022-00845-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00845-2