Abstract
Among the high-performance and engineering polymers, polyimides and the closely related polyetherimide (PEI) stand out by their capability to react with nucleophiles under relatively mild conditions. By targeting the phthalimide groups in the chain backbone, post-functionalization offers a pathway to adjust surface properties such as hydrophilicity, solvent resistance, and porosity. Here, we use ultrathin PEI films on a Langmuir trough as a model system to investigate the surface functionalization with ethylene diamine and tetrakis(4-aminophenyl)porphyrin as multivalent nucleophiles. By means of AFM, Raman spectroscopy, and interfacial rheology, we show that hydrolysis enhances the chemical and mechanical stability of ultrathin films and allows for the formation of EDC/NHS-activated esters. Direct amidation of PEI was achieved in the presence of a Lewis acid catalyst, resulting in free amine groups rather than cross-linking. When comparing amidation with hydrolysis, we find a greater influence of the latter on material properties.
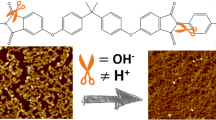
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surface functionalization is an extremely valuable approach to adapt the interaction of a polymeric material with its environment without modifying the behavior of the material itself. Adjusting the surface properties and functions of polymers is common in nearly every application, from technical foils via membranes to biomedicine, sensors, and electronic devices [1,2,3,4,5]. In general, surface modification can be achieved by either changing the chemical composition of the surface layer, or by adsorbing or blending with functional entities [6]. Chemical modification can be considered as more permanent and robust modification; however, most technical polymers are chemically inert. Their surface modification is typically based on or preceded by high energy or highly oxidative treatments such as plasma, UV-light + O2, flames, or chromic acid to create reactive hydroxyl or carboxyl groups [7, 8]. Clearly, such harsh procedures have a high chance of causing chain cleavage, with a profound impact on surface morphology and chemical and mechanical resistance.
A class of high-performance polymers that can be surface modified without oxidative pretreatment are polyimides (PIs) and the more easily processable polyetherimide (PEI) (Fig. 1a) [9]. Owing to the presence of phthalimide groups in the backbone, these polymers can react with nucleophiles in a polymer analogous reaction, meaning that the chain length remains unaffected. In addition to their excellent mechanical and thermal properties, PEI and PIs can be processed into porous devices such as particles [10] and membranes [11]. For such rather delicate structures, the possibility of a chemical post-functionalization under mild conditions is especially interesting, e.g., to enhance solvent resistance via cross-linking or to reduce adsorption of solutes through hydrophobic interactions via hydrophilization.
(a) Polyetherimide (PEI) (b) Experimental sequence: The polymer is compacted at the air–water interface. Next, reactants are injected and the layer is observed with interfacial rheology. Then, the layer is transferred to a solid substrate or grid. The solubility of the layers on solid substrates is assessed by washing with DMF, chloroform, and water. (c) Overview over four investigated functionalization procedures: (I) Direct amidation with and without Lewis acid catalyst. (II) Hydrolytic ring opening, (III) 3-step mechanism from hydrolytic ring opening via EDC/NHS activation of the generated carboxyl groups to amidation with (IIIa) EDA or (IIIb) TAPP. If the same amine reacts with two or more chains, cross-links are formed.
The by-far most important nucleophile-based surface functionalization procedures for PEI and PIs are aminolysis and alkaline hydrolysis. Ring opening of the phthalimide units with hydroxyl ions creates carboxyl groups and enhances the surface hydrophilicity, which is expected to enhance fouling resistance of membranes [12]. Aminolysis is typically carried out with multivalent amines [13,14,15]. Depending on the reaction conditions, aminolysis can either result in cross-linking [16], or in free amino groups, which can be used to attach further functional entities. A major challenge for any surface modification procedure is the characterization of the properties of the new material formed in the surface region. The signals recorded by most spectroscopic methods arise in large part from the underlying, non-functionalized bulk material. For PI and PEI hydrolysis and aminolysis, this problem is aggravated by the fact that the vibrational spectra of the reaction products are often very similar to the starting materials. A morphological characterization of the surface layer by atomic force microscopy (AFM) is straightforward for flat or nanostructured devices but highly challenging for microporous systems. Assessing the chemical and mechanical stability of the newly formed surface materials is important to judge the long-term behavior of a functionalized polymer but requires a chemical and mechanical analysis that is decoupled from the underlying bulk material. All of the aforementioned challenges can be solved when ultrathin films are used as model systems for the polymer surface. The Langmuir monolayer technique [17] is especially suited to replicate the porous structures formed by phase inversion or coagulation of PIs and PEI. Here, the film formation involves a transition from a good to a bad solvent, similar to a phase inversion or coagulation process, resulting in nanoporous ultrathin structures (1–3 nm thickness) [18]. Langmuir monolayers have the additional advantages that interfacial rheology can be used as a characterization technique to detect the influence of the functionalization on the mechanical properties, and that the films can be transferred to different substrates for further characterization.
In our previous study, we focused on the reaction of PEI with hydroxyl ions, which, under suitable conditions, leads to ring opening without chain cleavage [18]. Here, we focus on the functionalization with multivalent amines (Fig. 1c), either directly or in a process involving hydroxyl ions. As a first step, we investigate different approaches for a direct reaction with aqueous amine solution, where three different outcomes can be expected: In absence of pH control and at high amine content, the solutions are alkaline, and hydrolysis could be favored over amidation. With pH control and an excess of amines, a high number of free amine groups are expected, whereas an excess of polymer could promote network formation when a multivalent amine reacts with several chains. The direct amidation of imides can be promoted by Lewis acids (Fig. 1c I). Here, Yb(III)triflate was chosen, which has shown both good activity [19] and water stability [20].
A three-step procedure is investigated as an alternative approach for amidation of PEI (Fig. 1c II). This procedure starts by ring opening of the phthalimide groups at pH 12.5, followed by activation with water-soluble carbodiimide and conversion to activated esters. Then, the activated esters react with bivalent (Fig. 1c IIIa) and tetravalent amines (Fig. 1c IIIb). With appropriate stoichiometry, the multivalent amines can bridge the chains, resulting in network formation. However, network formation requires reactive collisions between amidated and non-amidated monomers. With bulk PEI having a Tg > 200 °C, a thermal activation is required. Here, we investigate whether the Tg of ultrathin PEI films is significantly lower than the Tg of bulk PEI by temperature-dependent interfacial rheology.
Our experimental procedure is shown in Fig. 1b. The film is formed by spreading droplets of diluted polymer in chloroform solution. After evaporation of the solvent, the PEI film is compacted and the reactants are injected in the liquid phase. The progress of the reaction is followed by interfacial rheology or surface potential measurements. The functionalized films are transferred to electron microscopy copper grids and silicon wafers. The transfer to copper grids serves as a simple test of mechanical stability, and the films on silicon are characterized by AFM and Raman spectroscopy while their solubility is checked by washing with different solvents. The properties of the differently amidated films are discussed in comparison to merely hydrolyzed films.
Materials and methods
Materials
Polyetherimide (PEI), potassium hydroxide (KOH, anhydrous grade), hydrochloric acid (HCl, 37 wt%, analysis grade), phosphate-buffered saline (PBS buffer; 10x concentrated), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), N-hydrosuccinimide (NHS), ytterbium(III)triflate (Yb(OTf)3), ethylenediamine (EDA), and tetrakis(4-aminophenyl)porphyrine (TAPP) were purchased from Sigma (Darmstadt, Germany) and used without further purification. For all experiments, water was purified with a MiliQ system (Merck, Darmstadt, Germany). Solid substrates were silicon wafers (MTI Corporation, Richmond, USA) with a 300 nm thermal SiO2 layer and hexagonal mesh transmission electron microscopy grids (TEM grids) (Cu, 700 mesh, mesh size ~ 18 μm; agar scientific, Stansted, UK).
General procedure for Langmuir monolayer experiments
Experiments were carried out on “medium” area Langmuir troughs with A = 243 cm2 (Biolin Scientific, Espoo, Finland) with a subphase capacity of 180 mL, except for surface potential measurements (see below). For rheology, the area was A = 223 cm2 due to the area of the bicone with radius r = 25.5 mm. The surface pressure was measured with metal Wilhelmy plates which were calibrated against air and pure water. The polymer was spread from a chloroform solution with c ~ 0.1 mg/mL. Typically, 18 μg was spread. Compression rates were in the order of 10 mm/min. Surface leveling tools from Biolin Scientific were used for all experiments to compensate for water evaporation. When liquids were added to the subphase, the same amount of liquid was withdrawn from the trough using a syringe or a micropipette, before the beginning of the measurement.
The surface activity of all EDC, NHS, EDA, Yb(OTf)3, and TAPP was checked by injecting the compounds into PBS buffer and recording the evolution of the surface pressure. For all compounds, in the concentration range used for the experiments, the surface pressure did not increase by more than 0.1 mN/m within 5 h.
Direct amidation of PEI layers
The reaction was carried out either on PBS buffer or on purified water. The PEI layers were compressed to a surface pressure of 2 mN/m, resulting in a nanoporous film with a thickness of 1–3 nm. For the non-catalyzed experiment without buffer, 0.5 mL EDA was injected. For the experiments with "titration", 0.2 mL of 0.9 mg/mL EDA in PBS was injected repeatedly. For the catalyzed experiment with “titration,” 0.1 mL of 1 mg/mL Yb(III)triflate in water was injected beforehand. This experiment was carried out both at room temperature and at 40 °C. For the catalyzed experiment with high catalyst content, the concentration of Yb(III)triflate was 5 mM. Here, 15 μL EDA was mixed with 1 mL of PBS buffer and injected below the layer. After reaction over night, the films were transferred to silicon substrates and copper mesh grids by the Langmuir Schäfer (LS) method.
Amidation of PEI layers via EDC/NHS activation
The PEI/chloroform solution was spread on a subphase containing KOH at a pH of 12.5 and compressed to a surface pressure of 2 mN/m after solvent evaporation. Compression was halted while the surface pressure increased due to hydrolysis. After hydrolysis for 3 h, the pH was adjusted to 5–6 by injecting the appropriate amount of HCl. We found that the pH distribution was uneven throughout the trough and, therefore, gave 30 min for equilibration. EDC and NHS were dissolved in PBS buffer (10 × concentrated) and injected below the layer to reach a subphase concentration of 0.05 mol/L EDC and 0.1 mol/L NHS. After 1 h of activation, the amines were injected. A: 15 μL EDA was mixed with 1 mL of 10× concentrated PBS buffer and injected below the layer. B: 100 μg TAPP, dissolved in 1 mL concentrated HCl and filtered, was mixed with 1 mL 10 × concentrated PBS buffer and injected below the layer. After reaction overnight at room temperature, the layers were transferred to Si-wafers and TEM grids with the LS method.
Surface potential
The surface potential, ΔV, was measured with a MicroSpot Surface Potentiometer (Kibron, Helsinki, Finland) consisting of a vibrating plate potentiometer coupled to a MicroTrough G2 from the same company, with dimensions 80 × 405 × 5 mm3 (W × L × D), available surface area of 280 cm2, and a subphase capacity of 200 mL. The vibrating plate was placed approximately 2 mm above the water surface. An internal height compensation of the surface potentiometer was performed against the bare surface before spreading the polymer solution. The surface potentiometer’s value was set to zero before deposition of the monolayer on the surface.
Optical microscopy and solvent washing
The efficacy of cross-linking was evaluated by washing the transferred films with chloroform, dimethylformamide, and water. Before washing, the films were annealed at 50 °C for several days to enhance the film–substrate adhesion and prevent the film from floating off. Films were immersed and agitated for 10 s in chloroform, dimethylformamide, and water. The effect of the washing procedure was checked in a reflected light optical microscope (Zeiss Axio Imager A1m, Carl Zeiss, Jena, Germany).
Rheology
Rheology was carried out with a bicone geometry on an MCR 502 Rheometer (Anton-Paar, Graz, Austria). The bicone had a radius of r = 25.5 mm and was immersed in a medium area Langmuir trough. The angle of the tip of the bicone was 166.8°. Measurements were carried out at controlled strain of 1% (assuming an edge-wall distance of 3.5 mm, see below) and an oscillation frequency of \(f=1\)/s. We did not use a ring around the bicone, because we observed that the layer was too stiff to effectively enter into a circular slit. To account for this, the diameter of the slit was set to 100 mm when calculating the interfacial moduli using the algorithm from the RheoCompass software (Anton-Paar). Yet, the results are mainly of qualitative nature.
Atomic force microscopy (AFM)
The AFM measurements of films on silicon wafers were carried out on a MultiMode 8 (Bruker Corp., Billercia, USA) in tapping mode. Bruker RTESPW 150 Tips were used. All images were flattened by subtracting the median of the differences between scanning lines and leveled by defining three points of equal height.
Raman scattering
For measurements of reacted PEI, multilayer films were prepared by transferring the layer 10 times to the same silicon wafer using the Langmuir Schäfer method. Spectra were obtained with the confocal Raman microscope SENTERRA (Bruker Optik GmbH, Ettlingen, Germany). Raman spectra were measured at room temperature with a 532 nm laser source at a power of 2 mW in the spectral range of 70–3700 cm−1 with a 50 × 1000 µm2 aperture. An objective with magnification of 50× was used to collect the scattered light. The charge-coupled device (CCD) exposure time was 1 s, and an average of 128 cycles was used to increase signal-to-noise ratio. Samples were fixed on a computer-controlled xyz stage allowing increment stepping down to 0.1 μm in all directions. Spectra analysis was performed with OPUS software (version 8.2, Bruker Optik GmbH). For baseline correction, a baseline was approximated to the spectrum with linear splines.
Results and discussion
When PEI is spread from a volatile solvent at the air–water interface at neutral pH, it forms ultrathin films consisting of small polymer aggregates with a nanoporous morphology and a thickness between 1 and 3 nm[18]. When the polymers react with multivalent amines, cross-links can be formed by interchain reactions. Such reactions require the chains to have frequent collisions. With PEI having a Tg > 200 °C, it seems plausible that chains remain frozen at temperatures that are obtainable in aqueous environments. Yet, in ultrathin films [21] and in the presence of water [22], Tg can be substantially lower. Here, we used interfacial rheology at different starting surface pressures (Fig. 2a) to assess if the presence of water and the confinement in ultrathin films are sufficient to enable a mobilization of the chains. The experiment was limited by the temperature that can be maintained in a Langmuir trough system (up to ~ 50 °C). Here, the layer only softens at a very low surface pressure of 2 mN/m and above 40 °C. It is not clear whether the softening is an effect of enhanced chain mobility, or the consequence of enhanced mobility of bigger chain aggregates, which are less jammed at lower surface pressure. Nevertheless, 2 mN/m was chosen as starting surface pressure for further experiments, firstly for the potential thermal activation of chain motion at 40 °C and secondly, because the interfacial moduli are relatively low at this pressure, meaning that small changes arising from chemical modifications can be detected more easily.
(a) Temperature sweeps of PEI Langmuir films at different starting surface pressures. Storage modulus (G′) in squares and loss modulus (Gʺ) in dots. The indicated surface pressures give the surface pressure at 20 °C. During heating, the recorded surface pressure increased due to lowering of the surface tension of water. (b) Reaction of PEI Langmuir films at room temperature. The first steep increase in viscosity is the compression to 2 mN/m. Black curve: Large excess of EDA (ca. 20,000 x) added at 110 min. No buffer was used, pH increased to 11. Red curve: "Titration", where ca. 10 x excess of EDA was added at the times indicated by the arrows. The pH was kept constant by PBS buffer.
The first set of experiments aimed at determining the effect of stoichiometry and carried out at room temperature (Fig. 2b). To examine the influence of an excess of amine, ethylenediamine (EDA) was injected below the layer to achieve a concentration of 0.25 wt% in the aqueous subphase as suggested in [16]. This corresponds to a ca. 20,000 x molar excess with respect to phthalimide groups in the layer. The high excess of diamine caused an almost immediate softening of the layer. Further addition of EDA up to a concentration of 0.75 wt% in the aqueous phase (data not shown) leads to increase in surface pressure together with a modest increase in viscosity. This behavior is typical for hydrolytic phthalimide ring opening of PEI, which was probably caused by the high pH of the diamine solution (pH ~ 11). Further experiments were carried out with pH control. When adding EDA in moderate amounts (up to ca. 30 × molar excess) at room temperature and with pH control, we found a slight increase in the layer stiffness, whereas upon further addition, the layer became softer (red curve in Fig. 2b). We infer that in the absence of catalyst and under excess of diamine, cross-linking can only occur to a very moderate degree.
Searching for suitable catalysts, Yb(III)triflate has been described as an efficient Lewis acid for catalyzing the amidation of imides [19], while also being water tolerant [20]. Here, we investigated the catalysis of the amidation at elevated temperature (40 °C, see Fig. 3a) and at room temperature (Fig. 3b).
Addition of Yb(III)triflate and EDA (a) at 40 °C and (b) at room temperature. The steep increase in pressure and modulus corresponds to compression of the layer to 2 mN/m. The yellow arrow denotes the addition of the Lewis acid whereas the gray arrows denote the addition of EDA, ca. 10x excess for each addition.
Addition of the dissolved catalyst to the pre-formed layers resulted in no discernible change of modulus or surface pressure (yellow arrows in Fig. 3). The concentration of the catalyst was only about 1 μM; however, the molar ratio between phthalimide groups and the catalyst was about 1:3. When adding small amounts of diamine at room temperature, the modulus increased while the surface pressure remained constant. In contrast, the modulus and also the pressure decreased when EDA was added at 40 °C (gray arrows in Fig. 3), potentially due to chain scission process through catalytic amide exchange. Therefore, for further functionalization experiments, room temperature was chosen.
Previously, we have shown that also in thin PEI layers, the phthalimide groups can be opened at pH > 12 [18]. The ring opening enables a carbodiimide-catalyzed amidation as an alternative pathway to functionalize PEI with multivalent amines. In this approach, the layers are hydrolyzed at pH 12.5 for three hours to generate carboxyl groups. As can be seen in Fig. 4, during this time, both storage modulus and surface pressure increase. The increase in surface pressure can be attributed to the negatively charged carboxyl groups. The increasing storage modulus conforms with the disappearance of the nanometer sized voids in the film (Fig. 6b + c). This enhances the number of chain contacts and probably also entanglements, especially if the negatively charged chains adopt more extended conformations in the aqueous system.
Three-step functionalization via hydrolytic ring opening, carbodiimide activation, and aminolysis. After spreading on a subphase with pH 12.5, PEI was compressed to 2 mN/m. During hydrolysis for ca. 3 h, surface pressure and storage modulus increased. At (1), HCl was added to lower the pH to 6. After ca. 30 min (2), EDC and NHS were added to activate the carboxylate groups. After 1 h (3), aminolysis was initiated by adding either TAPP (a) or EDA (b).
After hydrolytic ring opening, hydrochloric acid was injected to reduce the pH to 6 (step 1 in Fig. 4). It was observed that equilibration of the pH was the most difficult part of the reaction from an experimental perspective. Because the trough is very shallow at only 5 mm but rather long at about 35 cm, it is necessary to dilute and distribute the acid into small aliquots that are deposited throughout the trough. Upon neutralization, we consistently observed a decrease in surface pressure due to removal of negative charges. To further help the neutralization, EDC and NHS were mixed with a concentrated (10 x) PBS buffer before injection. Relatively high EDC and NHS concentrations of 0.05 mol/L EDC and 0.1 mol/L NHS were chosen based on surface functionalization reactions from the literature [23]. Upon activation and formation of the NHS-ester, the surface pressure increased again (Step 2 in Fig. 4), probably due to the steric requirements of the NHS groups. After 1 h of activation, the amines were injected (Step 3 in Fig. 4), again mixed with buffer. In the case of EDA (Fig. 4b), both storage modulus and surface pressure decreased, whereas with the tetravalent TAPP (Fig. 4a), the storage modulus increased while pressure dropped back to almost the initial value. The latter result was very promising, indicating a similar chain packing as at the beginning but with enhanced cohesive force.
Based on the observation that the carbodiimide-mediated amidation can have a pronounced effect on the PEI layer when high amounts of catalysts are used, we repeated the reaction with Yb(III)triflate catalysis using the concentration (c = 5 mM) rather than the molar ratio reported in the literature [19]. As an alternative characterization method, the surface potential was measured (Fig. 5a). Ca. 2 h after injection of EDA (ca. 500 × molar excess, gray arrow in Fig. 5a), the surface potential started to increase and finally almost doubled. This observation would be in line with the formation of amide and amine groups, which can be partially protonated.
(a) Surface potential and surface pressure measurement of PEI on a subphase with high Lewis acid content (5 mM). The arrow indicates where EDA (ca. 500 x molar excess) was added to initiate amidation. Reaction was carried out in PBS buffer. (b) Raman spectra of Langmuir Schäfer films on silicon wafers: PEI Langmuir film, single transfer (black); Multilayer stack (10 layers) of PEI Langmuir film prepared as shown in Fig. 4 (green and red); Multilayer stack (10 layers) of PEI Langmuir film after reaction with EDA under Lewis acid catalysis as shown in 5A) (blue). The spectra are normalized to have similar intensities at 1780 cm−1 and are vertically offset.
To further investigate the outcome of the reactions, transferred multilayers (10 times LS transfers to the same silicon wafer) were investigated by means of Raman microscopy (Fig. 5b). The most pronounced change of all spectra, independent of catalysis or activation, is the appearance of strong C–H stretching bands around 3000 cm−1. Importantly, for the EDC-activated samples, the CNC vibration at 1380 cm−1 is upshifted to about 1410 while the C=O vibration remains at 1780 cm−1, which is characteristic of imides. Taken together, the spectra suggest that for the EDC-activated amidations, the reaction has stopped at the NHS activation stage, as the three aforementioned bands are characteristic of NHS. The strongest bands of TAPP [24] at 1550 and 1240 cm−1 are not observed in the EDC/TAPP spectrum. The layer functionalized with high amounts of EDA and Lewis acid catalyst shows a relatively strong peak at 1650 cm−1 (yellow circle in Fig. 5b) indicating a successful formation of amide groups, which agrees with the strong C–H stretching arising from the methylene units of EDA. A broad shoulder between 3200 and 3500 cm−1 could indicate the presence of free amine groups; however, this intensity could also arise from vibrations of H2O or simply be an artifact of the background correction.
The spectroscopic analysis of the transferred films provides a different view on the outcome of the carbodiimide-mediated amidation than interfacial rheology and surface pressure curves (Fig. 4), where a pronounced influence of the amines on the moduli and the surface pressure was observed. The latter effects could be explained by the formation of adducts, which are clearly present in the case of TAPP (Fig. 6a).
AFM height images together with line profiles of films after reaction with (a) TAPP and (b) + (c) EDA. The blue lines in the images show, where the line profile was extracted. (a) and (c) show positons where the film was fractured whereas (b) shows the closed film. The film shown in (b) was prepared with EDC activation whereas the film shown in (c) was prepared with Lewis acid catalysis. Image size is 1 × 1 μm2.
The morphology, as an important surface feature with a high chance to be influenced by a chemical treatment, was characterized by AFM after the different amine functionalization procedures (see Fig. 6). From height images, we can identify clear differences depending on the reaction procedure. The layer, which was directly reacted with EDA on 5 mM Yb(III)triflate, was morphologically identical to unreacted PEI at that surface pressure (Fig. 6c). It consists of small PEI particles, separated by pores with widths on the order of 50 nm. In contrast, the layer, which was reacted with EDA after hydrolytic ring opening and EDC/NHS activation, is completely closed and has much smaller pores. This is likely an effect of the phthalimide ring opening as demonstrated previously. Finally, the layer which was incubated with TAPP after EDC/NHS activation, is covered by a dense film. The thickness of the layer increased by about 1.5 nm, which conforms to the dimension of a TAPP molecule. When combining this finding with the absence of TAPP in the Raman spectra, we infer that these molecules are not covalently bound. Rather, the carboxylate groups, which were not converted to NHS esters, could undergo ionic hydrogen bonds with the amines of some TAPP molecules. These adsorbates would then direct the assembly of further TAPP molecules at the polymer surface via π–π stacking [25]. This additional cohesive layer explains the increase of the modulus. Intensive washing with water prior to the Raman measurements probably removed this layer. Nevertheless, the AFM images show how post-functionalization can be used to tune the structure of a PEI layer from nanoporous to completely closed.
When combining our aforementioned observations, it seems rather unlikely that simply mixing and gently heating PEI with multivalent amines at significant molar excess causes a cross-linking of the chains. Yet, higher solvent stability of the reacted membranes was reported [16]. Here, we speculate that this effect can be a consequence of PEI hydrolysis at high amine concentration. The solubility of PEI after different functionalization procedures was further investigated on transferred films on silicon wafers. The presence of the layer was checked by optical microscopy (see Fig. 7 top row), where the film appeared slightly brighter than the underlying substrate, which is most apparent at cracks and scratches.
Optical bright-field microscopy of Langmuir Schäfer films. Top row: Single transfer to silicon wafer. Middle: Same film after washing with chloroform, dimethylformamide, and water. The scale bar has a length of 20 μm and applies for the middle and top row. Bottom: transfer to copper mesh grids, mesh size = 18 μm.
After annealing at 60 °C for several days to enhance the adhesion between film and substrate, the films were washed with chloroform, dimethylformamide, and water. The corresponding optical bright-field images are shown in the middle row of Fig. 7. While the unmodified film was washed off, a layer hydrolyzed at pH 12.5 overnight remained undamaged, showing clearly that hydrolysis reduces the solubility of PEI in these solvents. Consequently, the layers, which had undergone hydrolysis prior to EDC/NHS activation, remained unchanged. The layer, which was directly reacted with EDA at 5 mM Yb(III)triflate was mostly washed away, but some small islands of polymer remained. An alternative simple test of the impact of the functionalization procedure on the “real world” properties of PEI was carried out by transferring the layers to copper mesh grids (see Fig. 7 bottom row). At 2 mN/m, the ultrathin PEI film was not able to span those grids, but at a high surface pressure of 10 mN/m, there were some sections covered by a ruptured and back-folded film. The PEI layer, which was directly reacted with EDA in the presence of a Lewis acid, was not strong enough to span the grid. In contrast, a freestanding film was found on some sections of the grid after hydrolysis at pH 12.5, in agreement with the findings from AFM and interfacial rheology. Interestingly, the capability to form freestanding films was even greater after EDC/NHS activation and reaction with EDA and TAPP. In the latter case, this is expected as the stabilizing TAPP layer was not washed off.
Conclusion and outlook
We have investigated the surface functionalization and possible cross-linking of PEI with multivalent amines in ultrathin films and compared the effects to those of hydrolysis at pH 12.5. We found that the properties of PEI are very sensitive to hydrolytic ring opening. We were able to activate PEI hydrolyzed to form NHS esters and to achieve some degree of direct amidation with EDA in the presence of Lewis acid catalyst. We were not able to reliably confirm that reaction with multivalent amines establishes cross-links. We attribute this to the lack of chain mobility and the still unknown appropriate cross-linker concentration. Nevertheless, in hydrolysis and EDC/NHS activation, we have identified an efficient procedure to produce cohesive freestanding ultrathin layers (1–2 nm) with improved solvent resistance and activated carboxyl groups. A logical next step would be to optimize the cleavage of the NHS esters by means of more nucleophilic multivalent amines to produce freestanding 2D membranes with even greater resilience.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
M.L. Jue, R.P. Lively, React. Funct. Polym. 86, 88–110 (2015)
K. Iijima, H. Nagahama, A. Takada, T. Sawada, T. Serizawa, M. Hashizume, J. Mater. Chem. B 4(21), 3651–3659 (2016)
A.M.D. Santos, A.C. Habert, H.C. Ferraz, J. Mater. Sci. Mater. Med. 28(9), 131 (2017)
A. Shiohara, B. Prieto-Simon, N.H. Voelcker, J. Mater. Chem. B 9(9), 2129–2154 (2021)
M.C. Tanese, G.M. Farinola, B. Pignataro, L. Valli, L. Giotta, S. Conoci, P. Lang, D. Colangiuli, F. Babudri, F. Naso, L. Sabbatini, P.G. Zambonin, L. Torsi, Chem. Mater. 18(3), 778–784 (2006)
P. Fabbri and M. Messori, in Modification of Polymer Properties, edited by C. F. Jasso-Gastinel and J. M. Kenny (William Andrew Publishing, New York, 2017), pp. 109–130.
J.M. Goddard, J.H. Hotchkiss, Prog. Polym. Sci. 32(7), 698–725 (2007)
K.C. Khulbe, C. Feng, T. Matsuura, J. Appl. Polym. Sci. 115(2), 855–895 (2010)
X.-M. Zhang, J.-G. Liu, S.-Y. Yang, Rev. Adv. Mater. Sci. 46(1), 22–38 (2016)
S. Basu, M. Heuchel, T. Weigel, K. Kratz, A. Lendlein, Polym. Adv. Technol. 26(12), 1447–1455 (2015)
Y. Zhang, M. Zhong, B. Luo, J. Li, Q. Yuan, X.J. Yang, J. Membr. Sci. 544, 119–125 (2017)
P. Kanagaraj, A. Nagendran, D. Rana, T. Matsuura, S. Neelakandan, K. Malarvizhi, Ind. Eng. Chem. Res. 54(17), 4832–4838 (2015)
P. Phomdum, S. Gassara, A. Deratani, W. Chinpa, Chin. J. Polym. Sci. 36(10), 1157–1167 (2018)
X. He, A. Zhou, C. Shi, J. Zhang, W. Li, Sep. Purif. Technol. 206, 247–255 (2018)
W. Albrecht, B. Seifert, T. Weigel, M. Schossig, A. Holländer, T. Groth, R. Hilke, Macromol. Chem. Phys. 204(3), 510–521 (2003)
K. Vanherck, A. Cano-Odena, G. Koeckelberghs, T. Dedroog, I. Vankelecom, J. Membr. Sci. 353(1), 135–143 (2010)
J.Y. Park, R.C. Advincula, Soft Matter 7(21), 9829–9843 (2011)
R. Machatschek, M. Heuchel and A. Lendlein, J. Mater. Res. 36, 2987–2994 (2021)
C. Guissart, A. Barros, L. Rosa Barata, G. Evano, Organic Lett. 20(17), 5098–5102 (2018)
E. Keller, B.L. Feringa, Tetrahedron Lett. 37(11), 1879–1882 (1996)
H. Fischer, Macromolecules 35(9), 3592–3595 (2002)
L.S.A. Smith, V. Schmitz, Polymer 29(10), 1871–1878 (1988)
C. Wang, Q. Yan, H.-B. Liu, X.-H. Zhou, S.-J. Xiao, Langmuir 27(19), 12058–12068 (2011)
H. Wang, J. Xu, J. Wan, Y. Zhao, X. Zheng, J. Phys. Chem. B 114(10), 3623–3632 (2010)
R. Machatschek, P. Ortmann, R. Reiter, S. Mecking, G. Reiter, Beilstein J. Nanotechnol. 7(1), 784–798 (2016)
Acknowledgments
The authors acknowledge Manuela Keller for performing AFM measurements and Langmuir monolayer experiments. Daniela Radzik is acknowledged for performing Raman microscopic measurements.
Funding
This work was financially supported by the Helmholtz Association through program-oriented funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Machatschek, R., Heuchel, M. & Lendlein, A. Thin-layer studies on surface functionalization of polyetherimide: Hydrolysis versus amidation. Journal of Materials Research 37, 67–76 (2022). https://doi.org/10.1557/s43578-021-00339-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00339-7