Abstract
The world of biology created a wealth of complex materials intertwining order, disorder, and hierarchy. They are produced with minimal energy expenditures and display combinations of properties that surpass materials aimed to be perfectly ordered crystals or perfectly disordered glasses. De novo engineering of biomimetic materials with “impossible” combination of properties necessary for multiple technologies becomes possible considering complexity as a design parameter but this methodology lacks foundational principles. This article delineates the concept of complexity in the context of materials science. It examines the pathway to quantitative complexity–functionality relations and explores pragmatic approaches to scalable complex materials guided by discrete mathematics of nanoassemblies from imperfect components.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The need for complexity in materials engineering originates from the hard challenges posed by environmental, economic, and social boundary conditions for their design. Sustainable materials must exhibit a difficult-to-achieve balance of physicochemical properties, such as strength, conductivity, transparency, recyclability and environmental robustness, to satisfy seemingly unrealistic requirements from multiple technologies while minimizing their environmental footprint. It is becoming increasingly clear that traditional crystalline materials (characterized by perfect order) and amorphous materials (characterized by simple uncorrelated disorder) cannot address these needs. Learning from biology, incorporating complexity into materials design by combining order, disorder, and hierarchical organization enables the most advanced and adaptable systems in Nature – those of living organisms. To increase the complexity of materials from accessible abiological components, whether or not directly repeating structural patterns found in living creatures – is a pragmatic pathway to new materials with enhanced performance while reducing resource consumption. A wide range of biological and abiological components can also self-assemble further reducing the energy footprint. Designing for complexity rather than structural perfection is, therefore, pivotal for a sustainable future.
Inorganic nanoparticles (NPs) are central to the subject of complexity because they (1) have a universal ability to self-assemble; (2) possess essential properties such as electric conductivity, mechanical strength, and catalytic activity; (3) provide the unique opportunity to observe the emergence of complexity due to high electronic contrast; and (4) can be easily paired with a wide range of organic and biological nanoscale components to produce a nearly endless spectrum of hybrid materials also known as composites. As empirically found complex self-assembled nanostructures continue to benefit energy, environmental, water, biomedical, information and other technologies, the search for quantitative relations between complexity and functionality of nanostructures that can purposefully accelerate their design becomes the focal point of materials science.
The objectives of this perspective are threefold: (1) to define the notion of complexity in the context of materials, (2) to establish methodology(ies) of its quantification, and (3) to provide pathways to complex yet easily scalable and energy-conscious materials via self-organized nanostructures.
What is complexity?
The subject of complexity has many faces. It represents a ubiquitous yet vague notion that does not have a universal agreed-upon definition based on the physical description of matter. The earliest thought about complexity traces back to Aristotle, who defined complexity as “The whole is more than the sum of its parts” (Metaphysics, Vol. VII). Note that this is a function-based definition of complexity, which can provide intellectual guidance to the construction of complex systems including materials.
The first modern attempts to transition from colloquial to formal definitions of complexity are associated with the development of programming and information theory. The works of Kolmogorov,1 Solomonoff,2 and Chaitin3 in the early 1960s described complexity as the minimal algorithm required to fully reproduce an object and its information content. Although all three of these scientists worked on this subject independently, this concept is often referred to as Kolmogorov complexity; we will refer to it as algorithmic and information complexity or AIC. As applied to chemical structures and generally to matter, AIC is a minimal algorithm that deterministically encodes the elemental composition, spatial coordinates, and point-of-time dynamics of atoms in the object. Repeatable patterns found in crystals, dramatically simplify the algorithms describing their atomic organization, and thus decreases AIC. In its classical interpretation, randomness of the atomic configurations of the materials increases AIC.
Making the next step, Wolfram in 1984,4 and Grassberger in 19865 explored the dependences of complexity on information theory and algorithm design that can be potentially exploited in the practical calculations of AIC. This work was continued by Çambel in 1992,6 Kauffman in 1993,7 Gell-Mann in 1994,8 Lopez-Ruiz et al. in 1995,9 and Holland in 1995,10 who considered complexity as a derivative of theories of cellular automata, chaos, and fractals. While using AIC as a steppingstone, their papers and books dramatically changed the foundational interpretation of complexity. Considering randomness is one of algorithmic rule governing the organization of a system, Çambel, Kauffman, and Gell-Mann argued that complexity is a combination of order and disorder (COD). We note that this view agrees with physical realities of large atomic structures described by statistical mechanics. It also agrees with information theory because the information content of two fully random distributions of the same atoms regardless of their amount is identical despite the difference in their coordinates. We also note that perfect crystalline materials in the context of both COD and AIC are similarly noncomplex because, on one hand their structural organization can be encoded by a short algorithm and, on another hand, crystals do not contain disorder as the second essential component. The difference between COD and AIC becomes vivid when the system’s randomness increases gradually starting from the perfect order and evolving into complete disorder. Starting from noncomplexity of crystals, COD initially increases and then decreases as the positions of atoms become fully randomized. The same trajectory of the system leads to a monotonical increase of AIC. Another difference between COD and AIC is that in its classical formulation AIC is dependent on the number of atoms in the structure and, therefore, is an extensive parameter. COD, on the contrary, is an intensive parameter as long as the increase of the number of atoms does not reveal a new level or organization of the atoms and a new pattern combining order and disorder at larger scale. If such hierarchical organization is present in matter, COD should abruptly increase as physical dimensions of the system become larger than the characteristic scale of the new organizational patterns.
Discussion of complexity in both interpretations enabled exploration of the relations between complexity and “big” subjects, such as the emergence of life and evolution of the universe, stimulated by an organization of the Santa Fe Institute. Expanding the ideas of Aristotle, Kauffman,7 Grassberger, Holland, and Gell-Mann11 also introduce the idea of effective and function-based complexity, which is important in the context of materials design because it strengthens the connection between complexity and materials properties.
Elegant, instructive, and quantitative insights into the relationship between complexity and thermodynamics were also made. The direct connection between the complex multifractal systems of particles and their entropy and enthalpy were made by Stanley and Meakin in 1988.12 The relationships between statistical thermodynamics and complexity were expanded by Lopez-Ruiz et al. in 1995,9 who showed the rise of complexity as the system moves away from equilibrium. The pathway to complexity was further elaborated by Bak, Tang, and Wiesenfeld,13 who promulgated the idea of “self-organized criticality” (SOC), which is considered to be a mechanism for the emergence of complexity in matter with a large degree of stochasticity. In this case, the trajectory of an open system starts from noncomplexity of random distribution of atoms or other particles and evolves toward consistent self-correcting geometric patterns (e.g., sand piles) not crossing into formation of perfectly ordered crystals.
The interest in complexity continued between 1990 and 2000 and expanded to chemical and biological matter. These studies included brain and neuronal networks,14 biological organisms,15 chemical systems,16,17,18 and nanoscale structures.19
Complexity and functionality
Complexity with purpose
Let us consider now the rationale for the use of complexity as a design metric for materials. The best examples of amazingly complex and universally useful chemical structures excelling in both performance and longevity are biological composites. They can be found in all parts of the biosphere and are typically made from renewable earth-abundant nanoscale components, which makes a particularly good case for complexity in the context of sustainability. High-performance biological composites are exemplified by seashell nacre,20 tooth enamel,21 articular cartilage,22 tree roots23 (Figure 1), and animal bones24 (Figure 2). Their complexity manifests in sophisticated geometrical patterns, multiplicity of the components, abundance of interfaces, and multiple scales of organization. All these structural aspects are needed for them to perform their intended duties while reducing the energy burden on the organism and maximizing access to the material’s components. For instance, organizational motifs of bones span multiple scales from 10–10 m to 10 m with all of them being essential for their combination of functionalities that include lightweight load-bearing, stem cell differentiation, immune response modulation, and connectivity with soft tissues (Figure 2).25,26 At the atomic and nanoscale levels, bones are based on NPs with distinct crystallinity. 25 The NP shape is asymmetric and they are exceptionally polydisperse.26 The mesoscale organization of the bone-forming NPs in different projections includes mutual alignment and complex stochastic patterns with pronounced helicity (Figure 2c–d).26 The microscale and submillimeter scale organization display characteristic diameter of pores, asymmetry of niches, and thickness of pore walls.27 What emerges from an overview of these and other biomaterials as well as their human-made replicas, such as layered organic inorganic composites from nanocarbons,37 is that the organizational pattern and constitutive building blocks can be different, but the combination of order and disorder is universal.
Examples of hierarchical organization of complex biomaterials. Transmission (a–d) and scanning (e) electron microscopy images of bone structure at different scales: (a, b) constitutive hydroxyapatite nanoparticles; 25,26 (c, d) mesoscale assemblies of nanoparticles in different projections; 26 and (d) microscale organization of hydroxyapatite nanocomposite.27 The arrows in Figure 2c point to individual inorganic crystallites self-assembled into the complex composite biomaterial.
The omnipresence of the order–disorder combination (that can also be described as correlated or non-random disorder) and its direct connection to complexity as the design parameter for high-performance materials can be seen at the different scales of organization of load-bearing materials. Their architecture exhibits (Figures 1 and 2) several universal structural traits: (1) Atomic periodicity of NPs is paired with amorphous layer at their interfaces;28 disorder shows up at the molecular and nanoscale level when inorganic NPs are templated by organic components surrounding them, which is typical for biomineralization.29,30,31,32,33,34 (2) Inorganic nanocomponents of biomaterials display consistent anisotropy manifesting as axial asymmetry, but they are never the same size or shape; the mismatch at the interfaces is mitigated by the amorphous sections and the locally guided reorganization of atoms in the NPs and oriented attachment.35,36 (3) Mesoscale alignment of nanorods or nanofibers is distinct and common, but it is also variable and imperfect even for tissue segments with similar functional requirements. (4) Microscale and millimeter-scale patterns have nonrandom pore dimensions, but their percolating patterns and wall connectivity are seemingly disordered. Identical observations can be extended to all high-performance biomaterials.
One of the reasons for the universality of these structural traits is that the combination of order and disorder enables hierarchical organization, which would be associated with high thermodynamic penalties or outright impossible in perfectly ordered atomic, molecular, nanoscale, or microscale crystals. For example, filling a spherical void in a perfect crystalline material with perfect cubes is not possible without some imperfections at the interface. Likewise, the disorder is needed for the change of the structural patterns at different scales. The formation of an interconnected dendritic network of curved pores at nanometer, micron, and millimeter scales (Figure 2d) requires some break in the perfect space filling packing of nanoscale parallelepipeds of cellulose. The same is true for nanoscale rods and microscale pores in bone in Figure 2. Some of the best examples of high functionality disorder occur when it is present in otherwise crystalline materials, including the inclusions of amorphous calcium carbonate31 and calcium phosphate32 in load-bearing biocomposites of marine organisms. Similar purpose is associated with disordered organic layers between the partially ordered inorganic platelets in layered biological composites and nacre-like human-made materials.37 The disordered sections are necessary for mechanical properties of squid sucker ring33 and spider silk.34 More generally, the disorder facilitates the scale-dependent “switch” from one organizational pattern to another required for survival-critical physical properties. These properties are determined by organization of matter at different scales and include, for example, hardness, toughness, density, light-scattering and nutrient transport being affected by all the structural parameters present in Figure 2.
Note that the intricate multiscale organizations have, however, enthalpic and entropic penalties. Noncomplex materials, for instance, perfectly crystalline minerals or perfectly disordered glasses, may be more advantageous with respect to the free energy and component availability. Nevertheless, the materials with high complexity are the ones that make a difference between existence and nonexistence for living organisms. They support life because they excel not in only one task but in multiple ones at the same time. Nacre, enamel, bone, cartilage, etc., require complexity because they must display high strength, high toughness, high ionic selectivity, high flux of nutrients, low weight longevity, and selectivity in cellular adhesion – all at the same time. Monolithic blocks of calcium fluorophosphate, collagen, or their mixture exhibiting complete disorder or perfect order (crystallinity) will not provide the hardness, strength, and longevity of enamel.21 This property set requires it to be made from hydroxyapatite nanorods oriented along the dominant direction and interlaced with protein “cushions.”
Besides examples from Nature, there are numerous human-made materials, and more generally, materials systems, that are neither fully periodic nor random, which is essential for their performance, robustness, and manufacturing. They span a vast range of technologies from neuromorphic computers to desalination membranes.
Considering the prior studies of complexity in the context of “big questions,” a parallel can be made with other complex systems evolving under multiple boundary conditions modeled by Kauffman38 and Holland.10 Similarly, complexity in biomaterials emerges because they must perform multiple tasks and conform to multiple functional requirements that would otherwise be impossible to fulfill.
Thus, in the context of materials science complexity can be defined as purposeful performance-oriented structural organization of matter, combining order and disorder. This definition is applicable to biological and nonbiological materials, static or dynamic matter, and open or closed systems. It also incorporates atomic, nanoscale, mesoscale, microscale, and macroscale structures relevant to understanding the interactive and malleable hierarchy in living and technological systems.39 The combination of order and disorder makes possible combining different structural motifs at multiple scales, which de facto incorporates the notion of “The whole is more than the sum of its parts,” where at each scale, bringing together different parts of the material gives rise to new complex properties, maximizing functionality.
Quantification of complexity
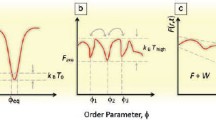
The approach to complexity as a parameter relating materials organization and performance can be described as a generic “Goldilocks” curve (Figure 3). Based on the necessity of materials structure to satisfy multiple requirements giving rise to multiple properties, the complexity and performance reaches a maximum somewhere between order (i.e., perfect continuous crystallinity) and disorder (i.e., uncorrelated isotropic randomness).8 The earliest version of such a curve was found in Huberman and Hoggs’ 1986 publication.40 Similar curves also appeared in subsequent publications by Lopez-Ruiz et al. in 1995,9 Edmonds in 1995,41 and Tononi et al. in 1998.14 Analogous conceptual antagonism between complexity and order also appears in art.42,43
COD or functional complexity peaks between ordered and disordered limits, defining the “Goldilocks” zone (solid blue line). AIC complexity monotonically increase with disorder (orange dashed line). NP, nanoscale particle. The scanning electron microscopy images in this figure are adapted with permission from Reference 71, Jiang et al., Science, 2020,51 and Kumar et al., Nature 2023.103 Other images are the original art forms of X. Mao.
We note that the axis of complexity in all the Goldilocks curves proposed in this field lack a quantitative assessment of complexity. This vagueness represents a major roadblock for future studies. Multiple approaches to quantify complexity have been proposed in the past.44 Some of the earliest were related to AIC that despite the limitations, can be used to approximate the left part of a Goldilocks curve. A string of symbols with minimal possible length for an ideal Turing computer to reproduce an object was a logical measure of AIC. One obvious problem here is that AIC is dependent on a programing language. A bigger problem, however, is that AIC is incomputable for any realistic object,45 which becomes particularly obvious in case of dynamic macromolecular structures relevant for chemistry or biology or nanoscale particles surrounded by water molecules. On the positive side, AIC can be related to entropy and entropy-related measure(s) of complexity were proposed by Grassberger5 and Rajaram and Castellani.46 AIC-inspired measures of complexity based on statistics integrated with thermodynamics were put forward by Crutchfield and Young in 1989 47 as well as by Lopez-Ruiz, Mancini, and Calbet in 1995.9
The examples of biomaterials shown in Figures 1 and 2 indicate that COD would be a better choice as a design parameter for materials engineering than AIC. Geometry-based measures of COD were proposed by Tononi et al. in 1994,14,48 Ay et al. in 2011,49 and Wolf et al. in 201815 Notably, all these approaches were inspired by biological systems. Cumulatively, they can be described as different methods to partition the actual physical system (Tononi, Ay) or its photographic image50 with subsequent quantification of the lost information due to the sectioning. Following Aristotle, the authors tested whether the complete system (object, image, etc.) has more information than a sum of its parts. In this regard, the emergence of new structural patterns combining order and disorder occurring at specific scale (i.e. partition) amounts to the emergence of additional functionalities differentiating one complex material from another. This approach capture the idea of and can theoretically lead to a quantitative Goldilocks curve.
Complexity of nanoscale assemblies
NPs can serve as a convenient model to decipher relationships between complexity and functionality because they can self-assemble into a large spectrum of structures combining order and disorder ranging from nearly perfect colloidal crystals with singular functionalities associated with the left side of the Goldilocks curve, to imperfect but multifunctional assemblies from polydispersed NPs in the middle part of the curve and eventually to nearly random agglomerates with limited functionalities for the right side of the curve. Additionally, the structures of inorganic nanoscale assemblies are convenient for various microscopy methods because of their higher contrast compared to organic materials. Finally, there are numerous NPs synthesized so far, and the family of composites based on their combinations and permutations between themselves and organic components is gigantic. As a conservative estimate, there are at least 100 reported nanoscale components. If they are to be tested for five different mixing ratios in a single polymeric matrix, there will be 2500 = 3.27·10150 test samples. Even with the proliferation of robotic laboratories and artificial intelligence, a universal approach to their design would significantly simplify the job of materials scientists.
Prior studies of complexity were not adapted to nanoscale structures in part because a methodology for the structural description of nanoscale systems that combined both order and disorder had not yet been defined.15,49 In one recent case, the architecture of nanoassemblies were analyzed using the toolbox of graph theory (GT).51 In this approach, NPs are represented as nodes, while the structural connectivity between them (both static and dynamic) is represented by edges (Figure 4). This general description can be universally applied across all material platforms with nanoscale organization. Importantly, the different shapes of the NPs, despite their variability in size, can be represented using simple GT formalisms. For example, nanospheroids, nanorods, and nanosheets (nanoplatelets) can be described as fully connected K1, K2, and K3 graphs. Chiral 3D structures with mirror asymmetric shapes can be represented by K5 graphs. All these representations of the constituent nanoscale building blocks correspond to the graphs of minimal complexity. Minimization of GT representations is needed to calculate complexity measures that would be comparable between different systems.
(a) Basic concept of graph theory (GT) description of assemblies of imperfect nanoparticles (NPs) represented as Kn complete graphs with increasing number of nodes depending on the shape and symmetry elements.50 (b) Transmission electron microscopy image of chains of CdTe NPs forming a network. Adapted with permission from Reference Tang et al., Science, 2002,36 and (c) their GT representation based on Kn formalism.51 Adapted with permission from51 Jiang et al., Science 2020.
In simple cases, GT models of nanoscale structures and nanoassemblies can be extracted from their TEM images exemplified by the particle chains in Figure 4b.36 For hierarchically organized structures with organization at multiple scales TEM and SEM images are required.52 High-resolution 3D reconstructions of nanoscale materials using electron microscopy or super-resolution optical microscopy are currently possible that will also provide the required information about the structural patterns. Note that graph descriptions of nanoscale structures can be applied to both size-limited nanoscale assemblies from NPs, nanorods and nanoplatelets, such as supraparticles, as well as to (semi)infinite materials, such as composites, gels, etc. The GT approach can be extended to continuous materials, such as nacre, enamel, and bone.51 For long-range structural patterns with high stochasticity, descriptions of nanoassemblies can be made in terms of random graphs and networks.53,54,55
The GT representations of different NPs in Figure 4 are given for the same level and scale of organization. The GT toolbox can also describe the hierarchical organized matter. For example, the atomic-scale structure of NPs, their interface with polymers, and the particle chains that form the polymer can be captured by deconvolution of single nodes into their own graphs built, for instance, with atoms as nodes. This can lead, of course, to very large graphs and in practice, it will be practical to collapse them into an additional NP characteristic – a nodal weight, wn. The statistical distribution of wn will incorporate the measure of order and disorder characteristic of the specific NPs. Similarly, one can add atomistic specificity to interparticle interactions by introducing edge weights, we, descriptive of nonrandom structural patterns of covalent and supramolecular bonds between them.
Once a representative graph of a nanostructure, or more generally, a material, is created, its complexity can be calculated. However, there is no universally accepted measure of complexity (see “Quantification of complexity”). Additionally, measures developed in the classical studies of complexity have not yet been applied to materials – nanoscale or otherwise. Several complexity measures could potentially be applicable to nanostructures. Among them are fractal exponents that describe self-similarity across the scales calculated from the GT representations.56,57,58,59,60 The quantitative parameters of resulting fractal exponents, that is, the prefactor and fractal dimension, df, are often associated with complexity61 but neither prefactor nor fractal dimension are actually measures of AIC or COD. The concept of fractality could be suitable for the description of hierarchical organization of materials when the patterns are repeatable at different scales. However, this is not true for the actual materials because the structural patterns do change (Figures 1, 2). Additionally, df, is typically calculated from original microscopy images, which also severely limits the range of scales essential for fractality. Under these circumstances, node-based multifractal spectra, f(α), extracted from GT representations developed by Bogdan62 are recommended. They describe a continuum of fractal dimensions changing depending on scale and location. The value of the Lipschitz-Hölder exponent, α, when f(α) reaches maximum is reflective of complexity and is promising for structure–property relations for complex materials.63,64,65 This approach can also be very effective for complex hierarchical graphs combining atomic, nanometer, and micrometer scales.
Another approach is the development of specialized indexes, such as the complexity index (CI).51,66 The calculation of CI is based on GT representations of nanoscale structures for which the complex organization of the material combining order and disorder are described based on the Kn formalism for nanoscale structures. The use of Kn enables one to abstract the structural imperfections and variability of the components that make up the material. CI for nanostructures is designed to identify the repeatable patterns of their organization. For example, the structure of NP chain can be represented by a simple graph formula in Figure 4c. The similarity with common chemical formulae is not accidental because they are indeed atomic graphs. The structure of more complex nanostructured materials, such as supraparticles from achiral nanosheets and nacre can be represented by the GT formulae in Figure 4a, where the platelets or platelet-like components are represented by K3 graphs. Two key differences with atomic graphs are that (1) the NPs can have variable shapes including those that are atypical for atoms (Figure 4a); (2) equality of sizes is not required and, unlike atoms, individual NPs are expected to have imperfections. Furthermore, these imperfections are beneficial for functionalities as can be observed for mechanical and electrical proprieties of layered composites from human-made and natural two dimensional (2D) materials.37,67,69
The repeatable organizational pattern of nacre or nacre-like human-made composites, that is, the stacking of nanoplatelets with organic layers in between, is uniformly described by a graph where K3 segments are interconnected by additional edges (Figure 5a). Such representation is universally applicable to the nacre-like composites from graphite oxide,67 graphene,68 clay,69 hydroxyapatite70 etc. The random distribution of nanoplatelets in supraparticles with repeatable nonrandom size is described by the loop with K3 segment inside (Figure 5b). GT representations of other complex particles both human-made (Figure 5c–d) and natural (Figure 5e) can also be built. CI is then calculated as the limited sum of (½)n progression for the total number of edges connecting each type of node to it nearest neighbors, next-nearest neighbors, second-next-nearest neighbors, etc. If there are more than one type of node, the CI for each of them are added together. Calculations of CI were first used when the materials with a combination of order and disorder, for instance, nacre with polydispersed platelets with stochastic yet nonrandom shapes were considered.51 Similarly, complex hedgehog supraparticles with randomly organized cores and a nonrandomly organized halo of spikes were successfully described by GT formalism where random portions of the particle with nonrandom diameter was described by loops.
Structural images (top) and graph theory representation (bottom) for various nanoassemblies. (a) Nacre-like composite represented stacks of graphite oxide nanoplatelets. Adapted from Reference 67. (b) Supraparticles from achiral Au–S nanosheets. (c) Kayak particle from achiral Au–S nanosheets. (d) Coccolith-like particles from chiral Au–S nanosheets. (e) Skeleton of the algae Syracosphaera Anthos containing chiral microsheets. Adapted with permission from Reference 51. Jiang et al., Science 2020.
As demonstrated by Jiang et al.,51 CI can serve as a measure of COD complexity being equal to 1 for randomized agglomerates and 6 for one-dimensional crystals (i.e., particle chains), while rising up to 24 for nacre-like composites and 87 for hierarchically organized particles (Figure 4). Working toward the quantification of the Goldilocks curve in Figure 3, the rise in CI can be directly associated with their functionality because it leads to acquisition of new properties controllable by organization at new levels and scales. The addition of atomic scale that describes the nanoparticles, nanorods, nanosheets, and the heterogeneity over the volume of the supraparticles will increase the degrees of freedom, the number of nodes in GT representations, and thus, the complexity. Concomitantly, this will also increase the number of properties that can be combined for a particle and the range of parameters that one property can be tuned independently of others. A recent proposal in this workspace was to perform real space renormalization on images and calculate the distances (i.e., dissimilarities) between images across consecutive steps of renormalization, and use the sum of these distances across the scales to define a multiscale structural complexity.50 This definition captures the variation of the structure as one zooms into smaller and smaller scales, which aligns with our definition of complexity in nanomaterials in terms of structural variation across scales.
GT representations of nanoassemblies enable applications of a versatile toolbox of discrete mathematics and network theory to characterize and predict properties of complex materials. For example, by assigning resistance and rigidity to edges of the graph, one can forecast electric and mechanical properties of the material across different scales. This opens the door to not only studying the relations between complexity and functionality, but also provide practical tools for materials design.
Pathways to complexity
Although many views on complexity have been proposed and many methods to predictively describe complex systems have been postulated, the actual pathways to real materials from imperfect components that exhibit intended complex behavior and an intended set of properties have been much less explored. The success of these studies will hold the key to combining complexity of materials with simplicity of manufacturing. In this section, we review several models that connect these characteristics with COD complexity.
Chirality
As often is the case with such difficult problems, some lessons can be learned from examining complex materials in Nature. Most of them are nanostructured, self-assembled, and chiral. Chirality, or mirror asymmetry, arises from the spatial arrangement and chemical distinction of atoms, molecules, NP, supraparticles, etc. This geometric characteristic and associated parameters introduce a uniquely practical dimension to the topic of material complexity. In chemistry and biology, it is widely accepted that chiral components self-assemble into intricate and sophisticated structures, which often exhibit properties that are very distinct from their initial components. Chiral molecules and particles can also be synergistically combined with nonchiral counterparts. The arrangement of chiral entities, such as molecules or NPs, can lead to the emergence of materials with complex helical or twisted structures.71
Understanding the relationship between the complexity of desirable materials and the chirality of their components provides a path to synthesize materials at large scale while carefully tuning their mechanical, optical, electronic, and biological properties. Although publications of empirical observations of complex materials with chiral molecules and particles are copious, the physicochemical mechanisms leading to their geometrical complexity are still puzzling. This is particularly true for nonuniformly sized nanoscale components that are essential for the scalability of certain processes. A direct inquiry into the dependence of chirality and complexity was made using a model system of supraparticles (SPs) from polydispersed gold thiolate NPs with the shape of nanoplatelets (K3). Because the surfaces of the NPs were coated with L- or D-cysteine (Cys) ligands, the flexible nanoplatelets twisted to become chiral. The resulting SPs then required K5 representation in the GT models.51 There was a clearly visible increase in the complexity of the self-assembled SPs as chirality increased as a result of changing the L/D-ratio from 1 to 10 (Figure 6). The calculation of CI values for SPs of different morphologies made at various temperatures and L/D-ratios demonstrated highly nonlinear growth of complexity as the chirality of the system increases.
(a) Product diagram observed for complex particles from chiral Au–S nanoplatelets. The blue and red phases on the top and bottom correspond to coccolith-like particles (aka chiral hedgehogs) with high complexity in Figure 5d. (b) Mapping of complexity index (CI) values on product diagram in (a). The highest (CI) values are observed for the part of the phase diagram where a triple point specific to critical states is observed. χ is the enantiomeric excess of chiral amino acids coating the Au–S platelets; tn is the temperature of the reaction. All images are adapted with permission from Reference 51 Jiang et al., Science 2020.
This dependence can be understood in the context of competitive restrictions on the short- and long-range self-assembly patterns that emerge for systems with different chirality. Electrostatic repulsion between the components engenders the nearly universal restrictions that impose limits on particle size, which is common for vesicles, micelles, inkjet droplets, exosomes, intracellular compartments, etc. In case of NP assemblies, electrostatic restriction vividly manifests both in nacre-like composites made from nanosheets by the layer-by-layer assembly72 and in SPs assembled from a wide range of NPs of different shapes.73 The complex nanoscale architectures emerge when restrictions are multiple, anisotropic, and competitive. What is essential is that energy gains and penalties associated with these restrictions on the self-assembly of building blocks must be comparable.
When none of the competing interactions and restrictions dominate, NP self-assembly acquires complex architectures to negotiate these conflicting requirements. For chiral NPs, the anisotropic restrictions associated with electrostatic repulsion are intertwined with those from hydrogen bonding, hydrophobic attraction, meta-ion coordination, and elastic deformation. The first order assessment of characteristic energies of these interactions when competitive restrictions result in complex architectures is 50–60 kJ/mol.51 Since the shape, charge, elasticity, hydrogen bond density, and composition of NPs change with L/D ratio and temperature, the characteristic energies also change. Thus, chirality provides the essential structural element at angstrom and nanometer scales for multiple restrictions to become competitive. Also important, chirality is a scaleless geometric property and, therefore, under favorable conditions it can be transferred from scale to scale and to higher scales, thereby ensuring competitive restrictions and complexity evolution as the size of the objects become larger.
The practicality of the chirality pathway to complexity can be highlighted by the fact that the polydispersity of NPs does not impede the formation of complex architectures. For Au-Cys NPs with extremely high polydispersity, the complexity of the resulting SPs is considerably higher than those formed from other NPs. Polydispersity may actually increase the complexity of the supraparticles, because the presence of smaller and larger NPs with stronger and weaker twists enables the growing assembly to select site-suitable NPs from the mixture.74 The uniqueness and tunability of the optical, chemical, colloidal, and potentially biological properties of these complex SPs suggest their broad application in asymmetric catalysis and polarization-based optoelectronics. The strong effect of chirality enables the scalable preparation and controllable preparation of complex particles in a sustainable manner and helps us to understand the origin of the astounding diversity and sophistication of biological nanocomposites (Figures 1 and 2). Further directions in this area may also include evaluation of the relations between COD complexity and chirality measures, exemplified by Hausdorff distance, Osipov–Pickup–Dunmur index, continuous chirality measure, and helicity measure. Also note that Harris, Kamien, and Lubensky showed that, instead of a simple “handedness” parameter, an infinite hierarchy of chiral moments can be utilized as chirality measures, which will need to be considered establishing these relationships by defining a specific path system reconfiguration taking place in concrete chemical systems.75
Spatiotemporal chaos
The term chaos refers to persistent random behavior resulting from deterministic equations with initial conditions. The logistic map is perhaps the simplest example of chaos where complexity emerges from one simple iterative mapping of \({x}_{n+1}=r {x}_{n}(1-{x}_{n})\) for \(r>{r}_{c}\approx 3.57\) through the onset of chaos, where orbits become irregular and small variations in the initial conditions change drastically over time (Figure 7a).76 In this case, AIC complexity is small because the pattern is generated from a simple equation. COD complexity is, nevertheless, high with cascading time scales, where temporal correlation function vanishes at long times, indicating that the information of the initial conditions is “forgotten.”
(a) Deterministic chaos from the logistic map. (b) Coexistence of spiral pattern and spatiotemporal chaos in a Belousov–Zhabotinsky reaction. Adapted with permission from Reference 79. (c) Cascading scales in turbulence. Adapted with permission from Reference 78. (d) Self-organized criticality nanoassembly of neuromorphic elements. Adapted with permission from Reference 102.
The more generalized concept, spatiotemporal chaos, describes extended systems involving many interacting degrees of freedom, and finite correlation in both time and space controlled by the time elapsed from the onset of chaos. Having “finite correlation in both time and space” is common in systems with stochasticity, such as statistical mechanics, but spatiotemporal chaos refers to how this phenomenon comes from nonlinearity, even when the system is fully deterministic. This concept is directly relevant for physical, chemical, and biological systems where complex spatial patterns arise from simple nonlinear dynamics rules.77 Other well-known examples include turbulence78 and spatial temporal phases in chemical pattern formation systems79 (Figure 7b–c).
The connections between spatiotemporal chaos and complex biosystems in Nature have been extensively discussed in prior studies. There is a high degree of certainty that spatiotemporal chaos can be a realistic pathway to complex materials. Similar to many cases of complex materials, this research direction remains largely unexplored for nanostructures and other materials.
Geometric frustration
In complement to electrostatic restrictions on assemblies of molecules and particles as can be observed in 2D assemblies of platelets37 and 3D assemblies of NPs,73 geometric frustration between unfitting geometric shapes can take the form of effective attraction and repulsion restricting the assembly patterns and, thus resulting in the increased complexity. Inability of the geometrical shapes to perfectly tile surfaces and fill 3D volumes without gaps or overlaps defines the commonly accepted understanding of geometric frustration, and leads to complex assemblies (Figure 8). For example, nontiling polygons assemble to trees, fibers, and bulk phases under different levels of surface tension.78 Geometric frustration has far-reaching influences in statistical mechanics and condensed-matter physics in many contexts from glass transitions, spin glasses, ergodicity breaking, frustrated magnetism, and quantum spin liquid states, giving rise to an abundance of complex phenomena. The geometric frustration in physical systems with criticality has also been considered as a plausible component of biological complexity,15 leading to both compartmentalization and rugged fitness landscapes with nonergodic evolution.
(a) Packing of two right-handed chiral molecules by fitting their “grooves.” Short- and long-pitched molecules led to opposite handedness at the next level.75 (b) Elastic strain of fiber bending builds up as twisted bundles grow.80 (c, d) Non-Euclidean crystals of geometrically frustrated tetrahedra particles and associated assemblies in Euclidean space84 in different projections.
Geometric frustration may cause and be caused by chirality (Figure 8a). A good example is the assembly of twisted bundles described by Grason and Bruinsma in 2007 (Figure 8b), where the relative twist between neighboring fibers in a bundle assembly leads to the overall helicity.80 The authors showed that accumulation of stress and self-limited growth are key outcomes of geometric frustration in self-assembly. More generally, geometric frustration can be viewed in a broad class of “shape incompatibility” problems, which show up in many different forms, from ill-fitting polygons (Figure 8c–d)81 to twisting cubes82 and frustrated tubes.83 Among these challenges, a new route to mathematically formulate and solve the problem was to find the non-Euclidean space where the shapes perfectly tessellate. This theory has been applied to tetrahedra, for which the ideal tessellation is the 600-cell characterized by positively curved 3D space (the 3-sphere, or S-3), which converges to assembled helicoids, in agreement with experiments (Figure 8d).84 The generality of the theory was illustrated by its application to icosahedra, which tessellates negatively curved space (the hyperbolic 3-space, or H-3), as well as rationalizing Euclidean space self-assembly.85
Far-from-equilibrium assembly
Kinetic processes far from equilibrium (FFE) generate particle-based structures combining order and disorder as exemplified by snowflakes and fractal nanoassemblies.86 These are distinct from aggregates formed during spatiotemporal chaos where nonlinear interactions lead to complexity. Fast growth and self-regulation via feedback, demonstrated by exhaustion of the particle supplies, are the main mechanisms for complex assembly in FFE processes. A variety of experimental systems of this sort have been explored, although a unified theoretical framework is still lacking, due to the challenging nature of nonequilibrium statistical mechanics.
A well-known case of FFE assembly is the dendritic growth of crystals (Figure 9a), originating from branching instabilities at growth interfaces, with snowflakes being the common example. The more disordered version of such branching assembly, diffusion-limited aggregation (DLA, Figure 9b), has been observed in a wide range of systems across ionic, atomic, polymeric, nanoparticle, and colloidal particle scales.87,88 An important case of DLA is the chemical conversion of ions into dendrites,87,88,89 which represents a safety problem in batteries and other energy-storage devices.90 Branching under FFE conditions also leads to multifractals. In addition, supramolecular polymerization in FFE conditions has also recently been shown to generate complex assemblies with rich behaviors, due to kinetic pathway dependence of the assembly.91,92
More recently, influenced by the blossoming field of active matter,93,94 using active driving at the particle level to control self-assembly has become a fruitful new research direction and led to the formation of interesting complex structures. Directional motion and rotation of the self-assembly building blocks have led to the discovery of a variety of active assemblies.95 Among their unique properties motility-induced phase separation was observed (Figure 9c).96
Supramolecular systems represent a wealth of complex structures.97 Organic monomers interconnected with multiple weak bonds are capable of assembly and polymerization can produce FFE states from a large variety of molecules.91,92 The dynamic nature of these assemblies with organizational patterns from angstroms to microns can provide them with a fast response to different environmental conditions and a variety of unique mechanical 97,98 and optical properties.99,100
Self-organized criticality
An interesting and potential general pathway to complex systems based on molecules and NPs is self-organized criticality (SOC). This phenomenon links nonlinearity with critical scaling, fractality, and robust complex behaviors, where nonlinear dissipative systems spontaneous evolve to criticality, showing long-ranged spatiotemporal correlations without fine-tuning. Frustrated assemblies, for instance, SPs, can be treated as quasi-equilibrium states,6 which enables the use of extensive statistical thermodynamics to select conditions for their formation. A strong indication that the SOC pathway to complexity is practical are the recent data on spontaneous formation of complex memristic101 and neuromorphic102 responses in NP and nanowire assemblies (Figure 7d).
To summarize, the study of pathways to complexity in the context of nanomaterials is still at its infancy. Predictions of complexity theories outline an exciting future where complex structures lead to functionality and adaptability. Tools to synthesize such systems, fundamentally understand their formation mechanisms, and predict their functionalities will be of great value.
Conclusions
The notion of “complexity” represents the intersection of the philosophical methodologies of deduction and induction. Deduction has prevailed in the methodology of physics research since the 19th century, where deterministic laws govern a wide variety of phenomena in nature. In contrast, induction is dominant for studies of “emergence” that has gained popularity since the second half of the 20th century, with discoveries of critical phenomena and nonlinear dynamics. Emergence of complexity in self-assembled systems encompasses stochastic behaviors governed by internal conflict between different comparable restrictions onto degrees of freedom for the system.51 While in some cases complexity can be predictably engineered and gradually built,37 we also need to be open to the possibility that in many cases the outcome interactions can be difficult to deduce because they are less deterministic, and the complex system above the atomic scale are inherently nonergodic.38
The concept of structural complexity is at the center of emergence, where a realm of fascinating phenomena arises even when the system components obey simple rules. Self-assembling NPs represent the missing link in emergence of complexity because they are at the intersection of scales when the chemical systems become strongly nonadditive105 and the collective, not pairwise, interactions drive the formation of structurally sophisticated assemblies. Translating this point to materials design, the interactions between the materials components do not need to be excessively complicated and contain multiple parameters to make complex materials. These interactions do need to be, however, comparable in energy and experimentally deducible.
Scientists now have the capabilities for fundamental and practical studies of complex materials, especially for nanoscale components, that satisfy many requirements and are experimentally convenient. Guided by GT and other parts of discrete mathematics, enabling quantification of complexity, one can aim at better understanding of control and utilization of order, disorder, and hierarchy for specific property sets establishing the links between complexity and functionality. Integration of GT and network science with physics-based property descriptions106 will lead to unified methodologies for establishing specific graph-property relations for mechanical, thermal, electric, optical, chemical, and biological properties taking advantage of stress, heat, charge, and mass transfer through the nanoscale networks. We expect that dynamic systems based on polydispersed NPs will be created demonstrating gradual emergence of the hierarchical 3D patterns spanning multiple scales with direct utility in energy technologies, optoelectronics, membrane science, and biomedical applications.
References
A.N. Kolmogorov, Probl. Inf. Transm. 1, 1 (1965)
R.J. Solomonoff, A Preliminary Report on a General Theory of Inductive Inference, Vol. 131 (1960)
G.J. Chaitin, J. ACM 16(3), 407 (1969)
S. Wolfram, Commun. Math. Phys. 96(1), 15 (1984). https://doi.org/10.1007/BF01217347
P. Grassberger, Int. J. Theor. Phys. 25(9), 907 (1986)
A.B. Çambel, Applied Chaos Theory: A Paradigm for Complexity, 1st edn. (Academic Press, New York, 1992)
S.A. Kauffman, The Origin of Order, Self-Organization and Selection in Evolution (Academic Press, New York, 1993)
M. Gell-Mann, The Quark and the Jaguar: Adventures in the Simple and the Complex (W.H. Freeman, New York, 1994)
R. Lopez-Ruiz, H.L. Mancini, X. Calbet, Phys. Lett. A 209, 321 (1995)
J.H. Holland, Hidden Order: How Adaptation Builds Complexity (Helix Books, Reading, 1995)
M. Gell-Mann, Complexity 1, 1 (1995)
H.E. Stanley, P. Meakin, Nature 335(6189), 405 (1988). https://doi.org/10.1038/335405a0
P. Bak, C. Tang, K. Wiesenfeld, Phys. Rev. Lett. 59(4), 381 (1987). https://doi.org/10.1103/PhysRevLett.59.381
G. Tononi, G.M. Edelman, O. Sporns, Trends Cogn. Sci. 2(12), 407 (1998)
Y.I. Wolf, M.I. Katsnelson, E.V. Koonin, Proc. Natl. Acad. Sci. U.S.A. 115(37), E8678 (2018). https://doi.org/10.1073/pnas.1807890115
M. Randić, D. Plavšić, Int. J. Quantum Chem. 91(1), 20 (2002). https://doi.org/10.1002/qua.10343
M. Randic, D. Plavsic, Croat. Chem. Acta 75(1), 107 (2002)
T. Böttcher, J. Chem. Inf. Model. 56(3), 462 (2016). https://doi.org/10.1021/acs.jcim.5b00723
N.A. Kotov, F. Stellacci, Adv. Mater. 20(22), 4221 (2008). https://doi.org/10.1002/adma.200803045
S. Blank, M. Arnoldi, S. Khoshnavaz, L. Treccani, M. Kuntz, K. Mann, G. Grathwohl, M. Fritz, J. Microsc. 212(3), 280 (2003). https://doi.org/10.1111/j.1365-2818.2003.01263.x
B. Yeom, T. Sain, T. Lacevic, D. Bukharina, S.H. Cha, A.M. Waas, E.M. Arruda, N.A. Kotov, Nature 543(7643), 95 (2017)
E.H. Lim, J.P. Sardinha, S. Myers, Arch. Plast. Surg. 41, 231 (2014)
J. Ďurkovič, F. Kačík, H. Husárová, M. Mamoňová, I. Čaňová, New For. (Dordrecht) 51(1), 119 (2019). https://doi.org/10.1007/S11056-019-09723-Y
L. Bergström, E.V. Sturm, G. Salazar-Alvarez, H. Cölfen, Acc. Chem. Res. 48(5), 1391 (2015). https://doi.org/10.1021/ar500440b
N. Roveri, B. Palazzo, M. Iafisco, Expert Opin. Drug Deliv. 5(8), 861 (2008). https://doi.org/10.1517/17425247.5.8.861
H.P. Schwarcz, D. Abueidda, I. Jasiuk, Front. Phys. 5, 39 (2017). https://doi.org/10.3389/fphy.2017.00039
D. Kytyr, V. Petranova, O. Jiroušek, “Assessment of Micromechanical Properties of Trabecular Bone Using Quantitative Backscattered Electron Microscopy,”13th Bilateral Czech/German Symposium (2012), vol. 1, pp. 119–122
Z. Hewei, L. Shaojia, W. Yan, Y. Yonghai, G. Mingrui, L. Yangbei, Z. Xiaolong, D. Xuliang, Science 375(6580), 551 (2022). https://doi.org/10.1126/science.abj3343
W. Huang, D. Restrepo, J.Y. Jung, F.Y. Su, Z. Liu, R.O. Ritchie, J. McKittrick, P. Zavattieri, D. Kisailus, Adv. Mater. 31(43), 1901561 (2019). https://doi.org/10.1002/ADMA.201901561
A. Khayer Dastjerdi, R. Rabiei, F. Barthelat, J. Mech. Behav. Biomed. Mater. 19, 50 (2013). https://doi.org/10.1016/J.JMBBM.2012.09.004
L. Addadi, S. Raz, S. Weiner, Adv. Mater. 15(12), 959 (2003). https://doi.org/10.1002/ADMA.200300381
J.C. Weaver, G.W. Milliron, A. Miserez, K. Evans-Lutterodt, S. Herrera, I. Gallana, W.J. Mershon, B. Swanson, P. Zavattieri, E. DiMasi, D. Kisailus, Science 336(6086), 1275 (2012)
M. Eder, S. Amini, P. Fratzl, Science 362(6414), 543 (2018)
S. Ling, D.L. Kaplan, M.J. Buehler, Nat. Rev. Mater. 3, 18016 (2018). https://doi.org/10.1038/NATREVMATS.2018.16
J.J. De Yoreo, P.U.P.A. Gilbert, N.A.J.M. Sommerdijk, R.L. Penn, S. Whitelam, D. Joester, H. Zhang, J.D. Rimer, A. Navrotsky, J.F. Banfield, A.F. Wallace, F.M. Michel, F.C. Meldrum, H. Cölfen, P.M. Dove, Science 349(6247), aaa6760 (2015)
Z. Tang, N.A. Kotov, M. Giersig, Science 297(5579), 237 (2002). https://doi.org/10.1126/SCIENCE.1072086
N.A. Kotov, I. Dékány, J.H. Fendler, Adv. Mater. 8(8), 637 (1996). https://doi.org/10.1002/ADMA.19960080806
S.A. Kauffman, A World Beyond Physics: The Emergence and Evolution of Life, 1st edn. (Oxford University Press, Oxford, 2019)
M.G. Mann, S. Lloyd, Complexity 2, 44 (1996)
B.A. Huberman, T. Hogg, Physica D 22(1), 376 (1986). https://doi.org/10.1016/0167-2789(86)90308-1
B. Edmonds, J. Logic Lang. Inf. 9(4), 419 (1995). https://doi.org/10.1023/A:1008363224791
P. Galanter, Technoetic Arts 14, 9 (2016)
P. Galanter, “Complexism and the Role of Evolutionary Art,” in The Art of Artificial Evolution, ed. by J. Romero, P. Machado, Natural Computing Series (Springer, Berlin, 2008), chap. 15. https://doi.org/10.1007/978-3-540-72877-1_15
R. Badii, A. Politi, Complexity: Hierarchical Structures and Scaling in Physics, 1st edn., Cambridge Nonlinear Science Series 6 (Cambridge University Press, Cambridge, 2008)
G.J. Chaitin, J. ACM 21, 403 (1974)
R. Rajaram, B. Castellani, Physica A 453, 35 (2016). https://doi.org/10.1016/j.physa.2016.02.007
J.P. Crutchfield, K. Young, Phys. Rev. Lett. 63(2), 105 (1989). https://doi.org/10.1103/PhysRevLett.63.105
G. Tononi, O. Sporns, G.M. Edelman, Proc. Natl. Acad. Sci. U.S.A. 91(11), 5033 (1994). https://doi.org/10.1073/pnas.91.11.5033
N. Ay, E. Olbrich, N. Bertschinger, J. Jost, Chaos 21, 037103 (2011). https://doi.org/10.1063/1.3638446
A.A. Bagrov, I.A. Iakovlev, A.A. Iliasov, M.I. Katsnelson, V.V. Mazurenko, Proc. Natl. Acad. Sci. U.S.A. 117(48), 30241 (2020). https://doi.org/10.1073/pnas.2004976117
W. Jiang, Z.B. Qu, P. Kumar, D. Vecchio, Y. Wang, Y. Ma, J.H. Bahng, K. Bernardino, W.R. Gomes, F.M. Colombari, A. Lozada-Blanco, M. Veksler, E. Marino, A. Simon, C. Murray, S.R. Muniz, A.F. de Moura, N.A. Kotov, Science 368(6491), 642 (2020)
D.A. Vecchio, S. Mahler, M.D. Hammig, N.A. Kotov, ACS Nano 15(8), 12847 (2021)
P. Erdös, A. Rényi, Publ. Math. Inst. Hung. Acad. Sci. 5.1, 17 (1960)
M.E.J. Newman, S.H. Strogatz, D.J. Watts, Phys. Rev. E 64(2), 26118 (2001). https://doi.org/10.1103/PhysRevE.64.026118
B. Bollobás, A. Thomason, North-Holland Math. Stud. 118, 47 (1985). https://doi.org/10.1016/S0304-0208(08)73612-0
J. Feder, Fractals (Springer, New York, 1988), pp.1–243
K.J. Falconer, The Geometry of Fractal Sets, Cambridge Tracts in Mathematics, Series No. 85 (Cambridge University Press, New York, 1985). https://www.amazon.com/Geometry-Fractal-Cambridge-Tracts-Mathematics/dp/05233054
D.-J. Wei, Q. Liu, H.-X. Zhang, Y. Hu, Y. Deng, S. Mahadevan, Sci. Rep. 3(1), 3049 (2013). https://doi.org/10.1038/srep03049
Y. Cho, J.-H. Shin, A. Costa, T.A. Kim, V. Kunin, J. Li, S.Y. Lee, S. Yang, H.N. Han, I.-S. Choi, D.J. Srolovitz, Proc. Natl. Acad. Sci. U.S.A. 111(49), 17390 (2014). https://doi.org/10.1073/pnas.1417276111
Y. Xue, P. Bogdan, Sci. Rep. 7(1), 7487 (2017). https://doi.org/10.1038/s41598-017-07209-5
V. Balaban, S. Lim, G. Gupta, J. Boedicker, P. Bogdan, Sci. Rep. 8(1), 12416 (2018). https://doi.org/10.1038/s41598-018-30654-9
X. Xiao, H. Chen, P. Bogdan, Sci. Rep. 11(1), 22964 (2021). https://doi.org/10.1038/s41598-021-02203-4
D.A. Vecchio, M.D. Hammig, X. Xiao, A. Saha, P. Bogdan, N.A. Kotov, Adv. Mater. 34(23), 2201313 (2022). https://doi.org/10.1002/adma.202201313
M. Cha, E.S.T. Emre, X. Xiao, J.-Y. Kim, P. Bogdan, J.S. VanEpps, A. Violi, N.A. Kotov, Nat. Comput. Sci. 2(4), 243 (2022). https://doi.org/10.1038/s43588-022-00229-w
M. Wang, D. Vecchio, C. Wang, A. Emre, X. Xiao, Z. Jiang, P. Bogdan, Y. Huang, N.A. Kotov, Sci. Robot. 5(45), eaba1912 (2020). https://doi.org/10.1126/SCIROBOTICS.ABA1912
M. Randic, J. Chem. Inf. Comput. Sci. 41(3), 639 (2001). https://doi.org/10.1021/ci000115m
N.A. Kotov, I. Dekany, J.H. Fendler, J. Phys. Chem. 99(35), 13065 (1995)
W. Cai, A.L. Moore, Y. Zhu, X. Li, S. Chen, L. Shi, R.S. Ruoff, Nano Lett. 10(5), 1645 (2010)
Z. Tang, N.A. Kotov, S. Magonov, B. Ozturk, Nat. Mater. 2(6), 413 (2003). https://doi.org/10.1038/nmat906
Y. Wu, X. Liu, Y. Li, M. Wang, RSC Adv. 4(84), 44427 (2014). https://doi.org/10.1039/C4RA07907H
J. Yan, W. Feng, J.-Y.Y. Kim, J. Lu, P. Kumar, Z. Mu, X. Wu, X. Mao, N.A. Kotov, Chem. Mater. 32(1), 476 (2020). https://doi.org/10.1021/acs.chemmater.9b04143
K. Ariga, Y. Lvov, G. Decher, Phys. Chem. Chem. Phys. 24(7), 4097 (2022). https://doi.org/10.1039/D1CP04669A
Y. Xia, T.D. Nguyen, M. Yang, B. Lee, A. Santos, P. Podsiadlo, Z. Tang, S.C. Glotzer, N.A. Kotov, Nat. Nanotechnol. 6(9), 580 (2011). https://doi.org/10.1038/nnano.2011.121
L. Tang, T. Vo, X. Fan, D. Vecchio, T. Ma, J. Lu, H. Hou, S.C. Glotzer, N.A. Kotov, J. Am. Chem. Soc. 143(47), 19655 (2021). https://doi.org/10.1021/jacs.1c05488
A.B. Harris, R.D. Kamien, T.C. Lubensky, Rev. Mod. Phys. 71(5), 1745 (1999). https://doi.org/10.1103/RevModPhys.71.1745
R.C. Hilborn, Pattern Formation and Spatiotemporal Chaos: Chaos and Nonlinear Dynamics: An Introduction for Scientists and Engineers (Oxford University Press, Oxford, 2000)
J.S. Langer, Rev. Mod. Phys. 52(1), 1 (1980). https://doi.org/10.1103/RevModPhys.52.1
P. Davidson, Turbulence: An Introduction for Scientists and Engineers (Oxford University Press, Oxford, 2015)
J. Liu, Z.-S. She, H. Guo, L. Li, Q. Ouyang, Phys. Rev. E 70(3), 36215 (2004). https://doi.org/10.1103/PhysRevE.70.036215
G.M. Grason, R.F. Bruinsma, Phys. Rev. Lett. 99(9), 098101 (2007). https://doi.org/10.1103/PhysRevLett.99.098101
M. Lenz, T.A. Witten, Nat. Phys. 13(11), 1100 (2017). https://doi.org/10.1038/nphys4184
A. Haddad, H. Aharoni, E. Sharon, A.G. Shtukenberg, B. Kahr, E. Efrati, Soft Matter 15(1), 116 (2019). https://doi.org/10.1039/C8SM01290C
D. Hayakawa, T.E. Videbaek, D.M. Hall, H. Fang, C. Sigl, E. Feigl, H. Dietz, S. Fraden, M.F. Hagan, G.M. Grason, W.B. Rogers, Proc. Natl. Acad. Sci. U.S.A. 119(43), e2207902119 (2022). https://doi.org/10.1073/pnas.2207902119
F. Serafin, J. Lu, N. Kotov, K. Sun, X. Mao, Nat. Commun. 12(1), 4925 (2021). https://doi.org/10.1038/s41467-021-25139-9
P.W.A. Schönhöfer, K. Sun, X. Mao, S.C. Glotzer, Phys. Rev. Lett. 131(25), 258201 (2023)
J.M. Köhler, J. Kluitmann, A. Knauer, ChemistryOpen 8(12), 1369 (2019). https://doi.org/10.1002/open.201900231
T.A. Witten, L.M. Sander, Phys. Rev. B 27(9), 5686 (1983). https://doi.org/10.1103/PhysRevB.27.5686
T. Vicsek, Fractal Growth Phenomena, 2nd edn. (World Scientific, Singapore, 1992)
K. Manthiram, Y. Surendranath, A.P. Alivisatos, J. Am. Chem. Soc. 136(20), 7237 (2014). https://doi.org/10.1021/ja502628r
S.-O. Tung, S. Ho, M. Yang, R. Zhang, N.A. Kotov, Nat. Commun. 6, 6152 (2015). https://doi.org/10.1038/ncomms7152
A. Sorrenti, J. Leira-Iglesias, A.J. Markvoort, T.F.A. De Greef, T.M. Hermans, Chem. Soc. Rev. 46(18), 5476 (2017). https://doi.org/10.1039/C7CS00121E
N. Giuseppone, A. Walther, “Out-of-Equilibrium (Supra)Molecular Systems and Materials: An Introduction,” in Out-of-Equilibrium (Supra)molecular Systems and Materials, ed. by N. Giuseppone, A. Walther (Wiley-VCH, Weinheim, 2021). https://doi.org/10.1002/9783527821990.CH1
M.C. Marchetti, J.F. Joanny, S. Ramaswamy, T.B. Liverpool, J. Prost, M. Rao, R.A. Simha, Rev. Mod. Phys. 85(3), 1143 (2013). https://doi.org/10.1103/RevModPhys.85.1143
S. Ramaswamy, Annu. Rev. Condens. Matter Phys. 1(1), 323 (2010). https://doi.org/10.1146/annurev-conmatphys-070909-104101
S.A. Mallory, C. Valeriani, A. Cacciuto, Annu. Rev. Phys. Chem. 69, 59 (2018). https://doi.org/10.1146/annurev-physchem-050317-021237
M.E. Cates, J. Tailleur, Annu. Rev. Condens. Matter. Phys. 6(1), 219 (2015)
T. Aida, E.W. Meijer, S.I. Stupp, Science 335(6070), 813 (2012). https://doi.org/10.1126/SCIENCE.1205962
L. Su, J. Mosquera, M.F.J. Mabesoone, S.M.C. Schoenmakers, C. Muller, M.E.J. Vleugels, S. Dhiman, S. Wijker, A.R.A. Palmans, E.W. Meijer, Science 377(6602), 213 (2022)
K. Maeda, D. Hirose, M. Nozaki, Y. Shimizu, T. Mori, K. Yamanaka, K. Ogino, T. Nishimura, T. Taniguchi, M. Moro, E. Yashima, Sci. Adv. 7(27), eabg5381 (2021). https://www.science.org/doi/10.1126/sciadv.abg5381
F. Xu, S. Crespi, G. Pacella, Y. Fu, M.C.A. Stuart, Q. Zhang, G. Portale, B.L. Feringa, J. Am. Chem. Soc. 144(13), 6019 (2022)
R.T. Sibatov, A.I. Savitskiy, P.E. L’vov, Y.O. Vasilevskaya, E.P. Kitsyuk, Nanomaterials (Basel) 13(14), 2039 (2023). https://doi.org/10.3390/nano13142039
A. Diaz-Alvarez, R. Higuchi, P. Sanz-Leon, I. Marcus, Y. Shingaya, A.Z. Stieg, J.K. Gimzewski, Z. Kuncic, Sci. Rep. 9(1), 14920 (2019). https://doi.org/10.1038/s41598-019-51330-6
P. Kumar, T. Vo, M. Cha, A. Visheratina, J.-Y. Kim, W. Xu, J. Schwartz, A. Simon, D. Katz, E. Marino, W.J. Choi, S. Chen, C. Murray, R. Hovden, S. Glotzer, N.A. Kotov, Nature 615, 418 (2023)
M. Spellings, M. Engel, D. Klotsa, S. Sabrina, A.M. Drews, N.H.P. Nguyen, K.J.M. Bishop, S.C. Glotzer, Proc. Natl. Acad. Sci. U.S.A. 112(34), E4642 (2015)
C. Batista-Silvera, R. Larson, N.A. Kotov, Science 350(6257), 1242477 (2015). https://doi.org/10.1126/science.1242477
N. Kotov, Mi. Wang, K. Whishant, V. Cecen, L. Zhao, Z. Zhong, L. Liu, Y. Huang, Research Square, (2013). https://doi.org/10.21203/rs.3.rs-2758299/v1
Funding
The authors are grateful for the support from the National Science Foundation (2243104, Center for Complex Particle Systems (COMPASS)), Office of Naval Research (MURI N00014-20-1-2479), the US Department of Defense (HQ00342010033, Newton Award for Transformative Ideas during the COVID-19 pandemic), and the Air Force Office of Scientific Research (FA9550-20-1-0265, N.K.).
Author information
Authors and Affiliations
Contributions
All authors conceived, discussed, wrote, and edited the text, references, and figures.
Corresponding author
Ethics declarations
Competing interests
There are no funding-related conflict of interest or other competing financial interests for any authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, X., Kotov, N. Complexity, disorder, and functionality of nanoscale materials. MRS Bulletin 49, 352–364 (2024). https://doi.org/10.1557/s43577-024-00698-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-024-00698-6