Attacking mercilessly when you are strong and keeping out of harm’s way when you are weak. That is the whole secret of successful fighting. Get your enemy at a disadvantage and never, on any account, fight him on equal terms.
(George Bernard Shaw, Arms and the Man)
Abstract
More than half a billion years ago in the early Cambrian period, there began an evolutionary arms race between molluscs and their predators, in which molluscs developed armor in the form of a biomineral exoskeleton—a shell—to avoid being eaten by predators that were developing jaws and other novel means of devouring them. The mollusc fabricates multiple layers of shell, each of a particular microstructure of a composite between an inorganic and an organic phase, which are the end result of more than 500 million years of coevolution with increasingly deadly predators. Molluscan biomineralization is an excellent case to study how a biological process produces a complex structure, because the shell is constructed as an extracellular structure in which all construction materials are passed out of the cells to self-assemble outside the cell wall. We consider what is known of the development of multilayer composite armor in the form of nacre (mother of pearl) and the other strong microstructures with which molluscs construct their shells.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
An evolutionary arms race from the Cambrian to the present day
When the first efficient mobile predators evolved, about 540 million years ago, they had a distinct advantage over their prey. The fossil record shows us that soon after the origin of mobile predators came the origin of shells, and since that time, natural selection has produced predators with progressively better means of capturing, subjugating, and eating their prey. This has produced a corresponding natural selection for molluscs and other prey to minimize the predator’s advantage in diverse ways, such as developing toxicity, escape methods (burrowing), camouflage, counteroffensive (retaliation), and armor. This deeply historical arms race has been well reviewed by Vermeij.1 Armor is the method of “keeping out of harm’s way” that we focus on herein.
The first molluscan shells, together with other mollusc-like shells of uncertain affinities, appeared early in the Cambrian period, ca. 539 million years ago (Ma).2 Most of these early molluscs were tiny—millimeter-sized—animals that took part in the dramatic diversification event known as the Cambrian explosion, a geologically short interval of time (ca. 25 Ma) during which many extant and extinct phyla appeared. This period marked the simultaneous origin in many animal lineages of skeletons of various mineralogies, including calcium carbonate (both calcite and aragonite), calcium phosphate, and silica, as well as agglutinated skeletons.3 The sudden appearance of skeletons of diverse composition in many different lineages suggests an external trigger other than an alteration of their common oceanic medium and a change in seawater chemistry would presumably favor one type of mineralogy over others. Instead, it is thought that the primary cause of animals developing external skeletons was the onset of predation. The fossil evidence is overall consistent with this hypothesis, including the following supporting observations: (1) the earliest signs of predation occur at the base of the Cambrian or just before;4 (2) many different types of fossil evidence of predation have been recovered from Cambrian rocks, including predatory appendages on fossil arthropods,5 drill holes6, bite marks;7 ingested prey preserved in the digestive tract of predators,8 and healed shell scars;9 and (3) shells, thought by many to be primarily a tool of defense,1 appeared in many different animal lineages during the Cambrian explosion3 and were made of diverse minerals and had different microstructures3 and so likely evolved independently in many clades.
Although predation levels were high in the Cambrian compared to the latest Precambrian, only a few types of Cambrian predators are well known (e.g., the arthropods Anomalocaris and Opabinia, with claws and jaw-like appendages, and the Priapulida, with introvert). Predator diversity, efficiency, and deadliness all appear to have increased in the post-Cambrian part of the Paleozoic, as did the efficiency of defenses. Post-Cambrian, early Paleozoic times saw the advent of two major predator groups: on one hand the jawed fishes, which originated in the Ordovician and radiated in the Silurian–Lower Devonian and on the other the eurypterid arthropods, large (some 2.5-m long) predators with claws. Both groups contained the first meter-sized jawed and clawed “sea monsters.” In addition, nautiloid and ammonoid cephalopods with chitinous beaks, which had appeared in the late Cambrian, became quite large, up to several meters in length, during the subsequent Ordovician period. Very large scars, up to 60 mm long, have been found in some Ordovician cephalopods,10 revealing the presence of truly large predators. The Ordovician also saw the first appearance of stelleroid echinoderms and scolecodonts (polychaete jaws). Predation pressure thus rose in the early to middle Paleozoic seas. Some shelled prey, brachiopods and crinoid echinoderms, developed defensive traits in the form of spines or thick thecae.11 This increase in predation pressure on molluscs through the middle Paleozoic can also be seen in the diversification of shell-crushing predators through this time period: durophagous (shell-breaking) arthropod and fish genera increased dramatically during the Paleozoic, from just one genus in the Upper Ordovician–Silurian to more than 50 genera of shell crushers in the early Carboniferous.11,12
Another major jump in predation pressure occurred during the Mesozoic era when many groups of invertebrates and vertebrates evolved into increasingly effective predators. Among the predatory invertebrates, it is worth noting the diversification during this period of fast swimming cephalopods, decapod crustaceans equipped with powerful claws, predatory drilling snails, asteroid echinoderms, and others. Within the vertebrates, several reptile groups—nothosaurs, ichthyosaurs, plesiosaurs, mosasaurs, pachypleurosaurs, crocodilians (more than 150 genera of crocodilians in the Jurassic–Cretaceous), turtles (including the largest sea turtle in history, Archelon, which reached more than 4 m in length), and shell-crushing placodonts—adopted an aquatic lifestyle and constituted the large predators of Mesozoic seas. In addition, two aerial groups, pterosaurs and birds, arose and preyed upon fish of the Mesozoic oceans. At the same time, the variety and diversity of fishes continued to increase, including the origin and initial radiation of teleosts, the largest group of modern fish, and a great diversity of sharks and rays. Their prey, in order to cope with this increasing predation pressure, adopted new defensive strategies: they became more mobile and/or developed defensive traits in the form of spines, ribs, and thick shells. Moreover, many groups of gastropods and echinoids developed the ability to burrow within the sediment (i.e., became endobenthic), whereas other groups (e.g., bivalves, polychaetes) improved their ability to burrow both in terms of intensity and depth. This dramatic stage of the arms race is called the Mesozoic marine revolution.13
The process of escalation has continued to the present day, with some differences, such as the replacement of diverse Mesozoic marine reptiles by Cenozoic-toothed marine mammals (e.g., sperm whales, dolphins, killer whales). And while, by some accounts, predation frequency is at present about the same or slightly less than in the Mesozoic,1,14,15 by other measures (e.g., the number of marine families specializing in predation by shell breakage, the number of predatory gastropod families), there has been a continued increase of predation intensity from the Mesozoic to today.1,16 In particular, for bivalve molluscs and other groups (e.g., echinoderms such as echinoids, polychaete worms), epifaunal life on the surface of the seabed has become hazardous in view of the number and diversity of predators present there. The number of classes of burrowing (infaunal) animals has increased from the Mesozoic to the Cenozoic, especially with regard to those with deeply burrowing species.1 In particular, the origin and radiation of predatory (including shell drilling) prosobranch gastropods16 during the Late Cretaceous correlates with a dramatic increase in the proportion of marine bivalves that are infaunal (burrowing) instead of epifaunal.17,18 In addition, the Cretaceous/Tertiary boundary saw the demise of important groups of epifaunal bivalves: the diversity of oysters became drastically reduced and the rudists became extinct near this boundary. Thus, since the Mesozoic, bivalves that hide (e.g., clams) have diversified, while epifaunal ones have suffered higher rates of extinction. In contrast to the bivalve approach, which has largely been of escape, the gastropod approach has been more about strengthening armor through spines, knobs, a thickened aperture, and a thicker shell. This could be because the costs for shell damage are higher in bivalves: in them, even a small break means chemical leakage and hence detection by predators, whereas gastropods can still seal off their shell after a small break at the lip.19 Those lineages that did not adapt to increasing predation efficiency by hiding needed other solutions and one of these was to strengthen the shell to repel attacks. With the demise of ammonoids at the Cretaceous–Tertiary boundary and with the exception of a few species of Nautilus, Cenozoic cephalopods have internal or no shells, thus being agile, fast and efficient, and mostly having predatory habits.
This process of escalation between shell-crushing predators and armored molluscan prey is nicely illustrated in Lake Tanganyika, a rift lake that formed relatively recently in geological terms (12 million years ago).20 West et al.21 demonstrated how crabs in this lake with larger chelae (claws) had a greater success rate of predatory attacks on snails and likewise snails that were larger, had a thicker aperture lip, or stronger shell structure had a greater resistance to such attacks. West and Cohen22 later showed that snails with added shell layers of crossed lamellar (up to four layers of this strong shell microstructure—see next) showed greater resistance to shell breakage by crabs in the lake.
Escalation has been an important fact in shaping the structure of the communities, being responsible, at least partly, for the increase in the mobility of the faunas, their physiological buffering, and the predator/prey ratio.23
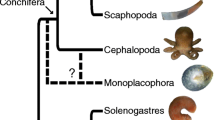
By inducing new life strategies (guilds) to cope with the increasing predation pressure, escalation has greatly contributed to both enlarging the ecological volume and to drastically increasing the diversity of those groups that have successfully coped with it; the other side of this argument can be seen in groups such as the famous trilobite arthropods, which lost their particular arms race and became extinct by the end of the Paleozoic. We should also comment on the cephalopods: most have lost or internalized their shells and become more mobile and, apart from Nautilus, other externally shelled cephalopods such as ammonoids have become extinct. Molluscs have become an extremely successful group in evolutionary terms, both as prey and as predatory animals—see Figure 1 for an example of extant molluscs possessing a mineralized exoskeleton—because they have been able to adapt themselves following Shaw’s maxim. Modern molluscs carry a long ancestry of success where for hundreds of millions of years every individual in their ancestral lines has successfully avoided predation prior to sexual maturity and much of this resistance had to do with the strength and efficiency of their shell.
Modern molluscan shells: An asterisk denotes the presence of nacre in some species of the class. Credits: Cephalopod, Manuae, CC BY-SA 3.0; Bivalve, Rachael Norris and Marina Freudzon, public domain; Monoplacophoran, The Trustees of the Natural History Museum, London, CC BY 4.0; Gastropod, Nick Hobgood, CC BY-SA 3.0; Polyplacophoran, Matt Knoth, CC BY 2.0; Scaphopod, George Manavopoulos, CC BY-NC 4.0.
Molluscan shell microstructures
If we examine the molluscan shell, we find it is made up primarily of calcium carbonate, a material that is not in absolute terms particularly strong. Calcite, one of the main crystalline polymorphic forms of calcium carbonate, comprises chalk, whereas the other common polymorph, aragonite, is a mineral encountered in an abiogenic context in caves as well as in present-day marine cements.24 Nonetheless, molluscs make use of calcium carbonate for their exoskeletons and they do so in such a way that their shells are much stiffer, stronger, and tougher than the pure mineral alone.
Mollusc shells are biominerals25 composed of assemblages of CaCO3 crystals with definite recurrent 3D arrangements, which are termed microstructures or ultrastructures (Figure 2). The type of microstructure depends, in the first place, on the mineral. The most typical calcitic microstructures are foliated composed of laths arranged into folia at a small angle to the growth surface, prismatic, formed of short prisms embedded in organic matter,26 and fibrous, similar to the foliated structure, but with fibers instead of laths as constituent units. Among the aragonitic microstructures, there is a prismatic counterpart to the calcitic prismatic microstructure; there is crossed lamellar, made up of vertical lamellae each consisting of aragonite needles that point oppositely in alternating lamellae; and there is nacre, the most famous microstructure, consisting of aragonite platelets that are arranged in a brick wall fashion. Its formation and structure are explained next in detail.
Each microstructure has a great majority of calcium carbonate with a small percentage of organic material: proteins and polysaccharides. The shell generally has a multilayer architecture, with several different microstructures to be found in a cross section through it. An archetypic nacreous mollusc has a shell composed of an outer organic layer (periostracum), an outer layer (which could be of calcite or aragonite; Figure 2), and an inner layer of aragonitic nacre that includes an aragonitic myostracum, a layer that becomes exposed on the inner shell surface at locations of muscle attachment (muscle scars); see Figure 3 for a sketch of bivalve molluscan anatomy and nacre structure.27
Although molluscs generally have two to three layers of different microstructures, more complex cases are known: for example, the Patellidae (Gastropoda) have up to five calcified shell layers with granular, crossed-foliated, myostracum, and crossed lamellar structures28 (pers. observations).
Nacre as a fibrous composite
Nacre is the molluscan biomineral microstructure that is least assimilable to abiotic structures and is the most studied, in part for that reason and in part because it makes up pearls and mother of pearl. The true fine-scale structure and mode of formation of nacre are just being uncovered. What follows is a summary of recent research on these aspects of nacre. Now that we are beginning to understand better how nacre is structured on a fundamental level, we can improve biomimetic and bioinspired methods that aim to replicate its great strength.
When the growth surface of nacre is viewed on the mesoscale, piles of tablets form a landscape of columns in gastropods,29 while steps or terraces of tablets are seen to give rise to arrangements of spirals, labyrinths, and target patterns in bivalves.30 If we zoom in to observe nacre on the microscale, it is found to be formed of flat bricks of crystalline calcium carbonate in its aragonite polymorph bounded above and below by an organic mortar—the interlamellar membrane—consisting of a proteinaceous matrix containing sheets of the polysaccharide chitin. This situation is sketched in Figure 3. In bivalves the structure is that of a brick wall, with tablets in each layer offset with respect to those in the layers above and below them, while in gastropods, the tablets are not offset, but are piled one on top of another. On looking in yet more detail, now at the nanoscale, we find that each brick is a composite of aragonite mineral incorporating protein fibers, while the mortar is a composite of chitin crystallites consisting of nanofibers of crystalline chitin embedded in a glycoprotein matrix. Nacre is thus a nonwoven nanofiber composite.
Not only is nacre a nanofiber composite, but it is moreover a self-assembled composite. Nacre is extracellular, being formed outside the cells of the mollusc and between the growth surface and the soft body of the organism there is a liquid-filled space, the extrapallial cavity, as we depict in Figure 3. All the components of nacre are secreted by the organism into this cavity, where they self-assemble in a hierarchical manner into nacre. An analogy will serve to illustrate the extraordinariness of this process. It is as if one empties clay, lime, sand, and water into a tub and finds that sheets of mortar form spontaneously within it, after which bricks grow themselves to fill the spaces between the mortars. That surrealist vision of how to build a brick wall is, however, exactly what a mollusc does when it builds nacre. As we illustrate in Figure 4, the construction hierarchy begins with chitin molecules, which polymerize and are extruded from the cells of the organism to form chitin crystallites of tens of polymer chains in the form of rods.27 These self-organize by their mutual interactions into a liquid crystal laid down layer by layer in a felt-like mesh. After the liquid-crystal layer has formed it becomes coated in protein that binds it and turns a liquid-crystal layer into a membrane, a new interlamellar membrane that is the mortar in the brick wall construction of nacre. At this point, the bricks are grown within the mortar: the liquid-filled space between interlamellar membranes is gradually mineralized by tablets of aragonitic calcium carbonate that grow incorporating within themselves fibrous proteins that previously were found in the liquid. Mineralization proceeds in gastropods with towers of growing tablets similar to piles of coins, the largest at the bottom and the smallest on top, as shown in Figure 5.29 In bivalves the mineralization takes a different route, forming terraces of growing tablets reminiscent of terraced hillsides in many traditional agricultures, as shown in Figure 6.30 The end result, however, is similar: a material nacre that is a hierarchically self-assembled fibrous composite that acts as part of the armor of the mollusc and is superior to anything human nanotechnology can produce today.31
Gastropod nacre: (a) Surface view of the nacre of Perotrochus caledonicus, showing the characteristic towered growth. (b) Section though the nacre of Clanculus jussieui; nacre towers grow within a complex arrangement of horizontal interlamellar membranes; the whole complex is topped by a thick surface membrane (SM). (c, d) Nacre of Gibbula pennanti. (c) Lateral view of the top of a tower; the last formed nucleus (top of the tower; arrow) is partly encased within the SM; the last formed interlamellar membrane (ILM) detaches from the SM. (d) Nuclei partly detached from the internal surface of the SM; the detachment damage (arrows) indicates that they were partly embedded within the SM. (e) Growing tablet; note the fibrous and porous aspect of the ILMs. (f) As in (e), after two plates have partly detached, the one below displays small protrusions though the pores of the ILM.32
Bivalve nacre: (a) Nacre of Nucula nitidosa; the growth fronts are terrace-like and here are covered by the interlamellar membranes; growth direction is toward the top left. (b) Surface view of the nacre of Pteria avicula; growth fronts are complexly arranged; the inset shows a tablet placed exactly at the origin of a spiral. (c) The same specimen; a detail of another area showing frequent target patterns and less frequent spiral shapes. (d) Transmission electron microscopy section through the nacre of Pinctada martensii; the two views show the evolution of two interlamellar membranes (IMs) from their initiation (upper view, white arrow) to the position of the first laid down tablet (lower view); the distance spanned is about 20 µm; the position of membranes is indicated with arrows; growth direction is toward the left; microvilli (MV). (e) Nacre of Anodonta cygnea with preserved interlamellar membranes; their texture is fibrous. (f) Same as in (e), showing putative nanopore (diameter ~20 nm).
Natural armor: Technological lessons from a mollusc
An engineer would not construct armor from such weak raw materials as those the mollusc uses. Evolution tinkers with what is available to it; in this respect it differs from technology, which, at least within some limits, generally makes a more global assessment of how to achieve a given end. The mollusc can obtain calcium and carbonate from its environment, but not, for instance, steel or some of the other materials humans would think of using for armor. Nature tends to build with a quite limited range of raw materials and moreover to do so at ambient temperature.33 Nonetheless, a mollusc can teach us useful lessons. Modern armor uses composite materials34 and has a multilayer structure both to prevent penetration and to dissipate impact energy. As mollusc shells are also composites with a multilayer structure, it is natural to ask whether molluscs discovered multilayer composite armor millions of years ago in the Cambrian. Let us consider some attributes of modern armor and see what parallels there are to be found in molluscan shell structures.
Multilayer structure
Mollusc shells have more than one layer. In general, the different layers of the shell tend to have distinct microstructures. The most common case is the superposition of microstructures with very different mechanical properties. Epibenthic bivalves tend to have an external calcitic or aragonitic prismatic layer together with a thicker inner foliated layer (in oysters) or nacreous layer (in pearl oysters). In both cases, the result is a tremendously flexible material that provides a tight closure for the two halves of the shell, needed to prevent predation and to avoid desiccation. In the case of the oysters, it constitutes a very useful strategy in those forms adapted to intertidal environments.35 The internal nacreous or foliated layer forms an efficient means of preventing a predator from penetrating to the soft tissue of the organism. The foliated layer is softer than the prismatic layer, but fractures in a localized manner, so preventing a general fracturing of the shell. However, different layers do not always have distinct microstructures. Many gastropod shells have different crossed lamellar layers in which the lamellae are oriented at up to 90° from each other.36 The superposition of layers of crossed lamellar in which the lamellae are arranged in straight lines constitutes an effective method for the deflection of fractures parallel to the lamellae.
In other cases, the advantage of multiple layers is of a chemical nature, as, for example, with the thick periostracum developed by freshwater bivalves and those molluscs living associated with deep-water hydrothermal vents, the so-called black smokers. The shells of chemical borers into carbonate substrates are protected by thick periostraca from their own acidic secretions. In all cases, the organic periostracum protects the shell from dissolution. In some groups of gastropods such as periwinkles, cold-water species have developed an external calcitic layer, which is much more resistant to dissolution at low temperatures than the aragonite that otherwise makes up the shell. One idea being explored in modern armor that ought to be examined to see whether it occurs in molluscan shells is a nanolayer change in material impedance to attenuate stress waves: the material properties in a layer change in a gradual way so as to tune the material such that impact stress is optimally dissipated. There are indications from observations of the way different microstructures in the shell blend into one another, rather than always having a sharp boundary that such nanolayer tuning could be found in molluscs.
Delamination and damage modes
That armor should dissipate energy as it is damaged by an impact is vital for its purpose. In modern multilayer armor, delamination is important with respect to the energy dissipation ability of the armor. Calcium carbonate, whether aragonite or calcite, is itself not a particularly strong solid, but the composites of calcium carbonate with an organic component as found in mollusc shell microstructures are far stronger. Studies have been made on how nacre behaves under mechanical damage,37,38,39 and it is found that there is considerable inelastic deformation before complete failure. During this process there can be observed processes contributing to the properties of nacre as effective armor, such as crack deflection, the delocalization of damage, plastic microbuckling or kinking, and viscoplastic deformation of the organic layers. For example, for a crack to propagate across a layer of nacre, it must deflect around each aragonite tile and must pull each out of the “wall” as the crack widens. During this process energy is absorbed by the debonding and by the shearing of the organic layer and by asperities on the tiles. Thus, the organic matrices provide just enough flexibility (i.e., allow some strain) to prevent catastrophic breakage. This gives nacre exceptional fracture resistance, as demonstrated by Currey,40 Jackson et al.,37 and Barthelat and Espinosa.41 Taylor and Layman42 and Currey43 performed comparative studies of shell microstructure strength, and both studies showed that nacre was the strongest. Nacre is energetically expensive and slow to grow43,44,45 and so there must be a strong selective advantage for it to outweigh such costs. We have shown that it is losing market share, at least in bivalves.25 On the other hand, as previously mentioned, other microstructures such as crossed lamellar structures are also well adapted to stopping and diverting cracks,46,47 making it particularly tough.48 Moreover, Gabriel49 demonstrated that other types of shell microstructure are stronger at resisting other forms of attack, such as by abrasion, acids, or organic solvents. This helps explain why deeply burrowing bivalves, which encounter much sand abrasion but few predatory attacks, are the only type of bivalve to have abrasion-resistant but weak homogeneous structures42,43,49
Sacrificial layers
In modern armor, a sacrificial layer is one sacrificed to save a layer beneath, ultimately to help prevent penetration of the whole structure. In molluscs, the outer layer of the shell can often be seen to serve this purpose. We have already mentioned how thick organic periostraca function in this way against chemical attack in some bivalves.
Adaptability
Molluscs have the ability to step up their defenses when necessary. Shells are energetically expensive, and molluscs show striking adaptability to strengthen them only when needed. For example, molluscs tend to strengthen those parts of the shell that are most in need of reinforcement, such as the aperture lip.1 In addition, Vermeij50 showed that dog whelks that live in areas with predatory crabs present have thicker, stronger shells than individuals in nearby environments that lack this predator. Also, on a larger scale, Vermeij51 has shown that more stable tropical regions show much higher levels of escalation than other regions, because of the long time for coevolution and high diversity of the tropics. Moreover, Avery and Etter52 describe how some species expand weaker, but presumably “cheaper” shell material (homogenous instead of crossed lamellar that co-occurs in the shell), presumably to get to a larger size, which has been shown in numerous studies to provide protection from predation.
Damage repair
A final point to be made about the material properties of the mollusc shell is that the mollusc is able to repair damage to its shell in a manner that should be the envy of those developing modern vehicle and body armor. If it is able to escape from a predator, a mollusc is able to repair even serious damage to the shell so that it is as strong as, or even stronger than, before.53 This shell remodeling is of necessity done from inside the shell,54 because, unlike bone, the biomaterial is extracellular and does not contain cells to remodel and repair itself, but through this method Nautilus, for instance, is able to produce both inner and outer shell layers during shell repair.55
Molluscan nanotechnology: There’s plenty of room at the bottom
From the Cambrian onward, molluscs have evolved their armor. As this armor is self-organized from the molecular scale upward and self-assembles from its constituents in breathtaking examples of virtuosity in nanotechnology, the mollusc shell provides the modern materials scientist with much food for thought. Materials scientists have already gained insight from the basic structure of nacre (see the review of nacre biomimetics in Reference 56). However, we are beginning to understand how nacre self-assembles,27 and we do not yet know how to imitate this self-assembly; a refined understanding of the fine-scale form of nacre reveals that we are far from truly replicating this complex shell microstructure.
More than 60 years ago, on December 29, 1959, Richard Feynman gave a talk entitled “There’s Plenty of Room at the Bottom: An Invitation to Enter a New Field of Physics” at the annual meeting of the American Physical Society at the California Institute of Technology. In it, he said, “What I want to talk about is the problem of manipulating and controlling things on a small scale. As soon as I mention this, people tell me about miniaturization, and how far it has progressed today. They tell me about electric motors that are the size of the nail on your small finger. And there is a device on the market, they tell me, by which you can write the Lord’s Prayer on the head of a pin. But that’s nothing; that’s the most primitive, halting step in the direction I intend to discuss. It is a staggeringly small world that is below. In the year 2000, when they look back at this age, they will wonder why it was not until the year 1960 that anybody began seriously to move in this direction.”57
What molluscs show us is that, more than 60 years later, we are still only taking primitive, halting steps in nanotechnology when we compare our efforts with the hierarchical fabrication from the molecular scale upward of sophisticated materials such as nacre that nature builds every day. Today, we have a technological arms race between those building ever-deadlier weapons and others engaged in finding ways to construct armor that will stop such weapons from achieving their target. As research is undertaken on composite materials for body and vehicle armor, it should not be forgotten that, as in so many other fields of technology, nature provides some useful lessons.
Data availability
Not applicable.
References
G.J. Vermeij, Evolution and Escalation: An Ecological History of Life (Princeton University Press, Princeton, 1987), p. 527
K.M. Cohen, S.C. Finney, P.L. Gibbard, J.-X. Fan, Episodes 36, 199 (2013)
S. Bengtson, S. Conway Morris, “Early Radiation of Biomineralizing Phyla,” in Origin and Early Evolution of the Metazoa, ed. by J.H. Lipps, P.W. Signor (Plenum Press, New York, 1992), pp. 447–481
S. Bengtson, Y. Zhao, Science 257, 367 (1992)
H.B. Whittington, D.E.G. Briggs, Philos. Trans. R. Soc. Lond. B 309, 569 (1985)
S.C. Morris, S. Bengtson. J. Paleont. 68(1), 1 (1994)
S. Conway Morris, The Crucible of Creation: The Burgess Shale and the Rise of Animals (Oxford University Press, Oxford, 1998), p. 242
H.B. Whittington, The Burgess Shale (Yale University Press, New Haven, 1985), p.168
C.B. Skovsted, G.A. Brock, A. Lindström, J.S. Peel, J.R. Paterson, M.K. Fuller, Biol. Lett. 3, 314 (2007)
B. Kröger, GFF 126(3), 311 (2004)
C.E. Brett, “Durophagous Predation in Paleozoic Marine Benthic Assemblages,” in Predator-Prey Interactions in the Fossil Record, ed. by P.H. Kelley, M. Kowalewski, T.A. Hansen (Kluwer Academic/Plenum Publishers, New York, 2003), pp. 401–432
P.W. Signor III, C.E. Brett, Paleobiology 10, 229 (1984)
G.J. Vermeij, Paleobiology 3, 245 (1977)
R.R. Alexander, G.P. Dietl, “The Fossil Record of Shell-Breaking Predation on Marine Bivalves and Gastropods,” in Predator-Prey Interactions in the Fossil Record, ed. by P.H. Kelley, M. Kowalewski, T.A. Hansen (Kluwer Academic/Plenum Publishers, New York, 2003), pp. 141–176
W.D. Allmon, J.C. Nieh, R.D. Norris, Palaeontology 33(Pt. 3), 595 (1990)
J.D. Taylor, N.J. Morris, C.N. Taylor, Palaeontology 23, 375 (1980)
A.R. Kabat, Malacologia 32(1), 155 (1990)
P.H. Kelley, T.A. Hansen, “The Fossil Record of Drilling Predation on Bivalves and Gastropods,” in Predator-Prey Interactions in the Fossil Record, ed. by P.H. Kelley, M. Kowalewski, T.A. Hansen (Kluwer Academic/Plenum Publishers, New York, 2003), pp. 113–139
G.J. Vermeij, Palaeontology 26(3), 455 (1983)
A.S. Cohen, M.J. Soreghan, C.A. Scholz, Geology 21(6), 511 (1993)
K. West, A. Cohen, M. Baron, Evolution 45(3), 589 (1991)
K. West, A. Cohen, Evolution 50(2), 672 (1996)
R.K. Bambach, A.H. Knoll, J.J. Sepkoski Jr., Proc. Natl. Acad. Sci. U.S.A. 99, 6854 (2002)
J.H.E. Cartwright, A.G. Checa, J.D. Gale, D. Gebauer, C.I. Sainz-Díaz, Angew. Chem. Int. Ed. 51(48), 11960 (2012)
J.H.E. Cartwright, A.G. Checa, C.I. Sainz-Díaz, J. Struct. Biol. 196(2), 65 (2016)
A.G. Checa, E. Macías-Sánchez, E.M. Harper, J.H.E. Cartwright, Proc. R. Soc. B 283, 20160032 (2016)
J.H.E. Cartwright, A.G. Checa, J. R. Soc. Interface 4, 491 (2007)
C. MacClintock, Bull. Peabody Mus. Nat. Hist. (22) (1967)
A.G. Checa, J.H.E. Cartwright, M.G. Willinger, Proc. Natl Acad. Sci. U.S.A. 106, 38 (2009)
J.H.E. Cartwright, A.G. Checa, B. Escribano, C.I. Sainz-Díaz, Proc. Natl. Acad. Sci. U.S.A. 106, 10499 (2009)
J.H.E. Cartwright, A.G. Checa, C.I. Sainz-Díaz, ACS Nano 14(8), 9277 (2020)
A.G. Checa, J.H.E. Cartwright, M.-G. Willinger, J. Struct. Biol. 176(3), 330 (2011)
U.G. Wegst, H. Bai, E. Saiz, A.P. Tomsia, R.O. Ritchie, Nat. Mater. 14(1), 23 (2015)
P.J. Hogg, Science 314, 1100 (2006)
E.M. Harper, A.G. Checa, Mar. Biol. 167, 78 (2020)
I. Almagro, J.H.E. Cartwright, A.G. Checa, E. Macías-Sánchez, C.I. Sainz-Díaz, Acta Biomater. 120, 12 (2021)
A.P. Jackson, J.F.V. Vincent, R.M. Turner, Proc. R. Soc. B 234, 415 (1988)
M.A. Meyers, P.Y. Chen, A.Y.M. Lin, Y. Seki, Prog. Mater. Sci. 53, 1 (2008)
K. Okumura, P.G. de Gennes, Eur. Phys. J. E 4, 121 (2001)
J.D. Currey, Proc. R. Soc. Lond. B 196, 443 (1977)
F. Barthelat, H.D. Espinosa, Exp. Mech. 47, 311 (2007)
J.D. Taylor, M. Layman, Palaeontology 15(1), 73 (1972)
J.D. Currey, “Shell Form and Strength,” in The Mollusca, vol. 11, ed. by E.R. Trueman, M.R. Clarke (Academic Press, San Diego, 1988), pp. 183–210
A.R. Palmer, Mar. Biol. 75, 287 (1983)
A.R. Palmer, Proc. Natl. Acad. Sci. U.S.A. 89, 1379 (1992)
J.D. Currey, A.J. Kohn, J. Mater. Sci. 11, 1615 (1976)
L.T. Kuhn-Spearing, H. Kessler, E. Chateau, R. Ballarini, A.H. Heuer, S.M. Spearing, J. Mater. Sci. 31, 6583 (1996)
H. Ji, X. Li, D. Chen, Sci. Rep. 7, 40043 (2017)
J.M. Gabriel, J. Zool. 194, 363 (1981)
G.J. Vermeij, Nature 299, 349 (1982)
G.J. Vermeij, Nature 260, 135 (1976)
R. Avery, R.J. Etter, Mar. Ecol. Prog. Ser. 323, 159 (2006)
J.A. Blundon, G.J. Vermeij, Mar. Biol. 76, 41 (1983)
A.J. Kohn, “Anti-predator Defences of Shelled Gastropods,” in Functional Morphology of the Invertebrate Skeleton, ed. by E. Savazzi (Wiley, New York, 1999), pp. 169–181
V.R. Meenakshi, A.W. Martin, K.M. Wilbur, Mar. Biol. 27, 27 (1974)
G.M. Luz, J.F. Mano, Philos. Trans. R. Soc. Lond. A 367, 1587 (2009)
R.P. Feynman, Eng. Sci. 23, 22 (1960)
Acknowledgments
A.G.C. acknowledges funding from Project No. PID2020116660GB-I00, funded by Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/ 501100011033).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cartwright, J.H.E., Checa, A.G. & Vendrasco, M.J. Arms and the mollusc: An evolutionary arms race has produced armor based on molluscan biomineralization. MRS Bulletin 49, 71–79 (2024). https://doi.org/10.1557/s43577-023-00594-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-023-00594-5