Abstract
Complex concentrated alloys (CCAs) are materials comprising three or more elements in similar proportions and possessing structural but no chemical long-range order. Fascination with CCAs has grown over the last 20 years and to date, CCAs have opened a new materials design paradigm and horizon for discovery of materials to meet the demands of applications in aggressive environments. Understanding the fundamental mechanisms controlling their response, however, is challenging due to the chemical and structural variations that wildly fluctuate over fine atomic and nanoscales. This issue focuses on the experimental, computational, and theoretical investigations that aim to uncover phenomena and processes determining the structure, kinetics, mechanics, or deformation mechanisms in CCAs at the atomic scale. At the atomic scale at which they operate, chemical short-range ordering can be influential. This issue further addresses the capabilities, as well as the debatable need, to characterize, predict, and relate short-range ordering to material performance. Collectively, the articles in this issue highlight the insights, understanding, and experimental and computational tools that attempt to create property-tunable CCAs “from the atom up” by treating short-range ordering and engineering atomic-scale mechanisms.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the classes of materials used in load-bearing applications, metallic alloys are used in the largest volumes, despite typically possessing the highest density. Yet, due to their ability to maintain strength even after yielding or being reshaped, they remain the structural material class of choice for the harsh environments anticipated in next-generation energy, space, and aerospace applications. With the introduction in 2004 of complex concentrated alloys (CCAs), a new alloy family, came the exciting potential to achieve the exceptional boosts in strength, and hence concomitant reductions in volume needed.1,2,3 CCAs also display a robustness in extreme high-temperature conditions, not possible with traditional alloys.4,5 With an eye toward future needs, the last 20 years have seen a growing fraction of alloy research shift focus and resources to CCAs.6
As the name suggests, CCAs are fundamentally distinct from traditional dilute alloys in chemical composition and concentration. Traditional alloys consist of a base metal (the principal element) and small amounts of one or several solutes (minor elements), with the alloy adopting the lattice structure of the principal element. For over a century, alloy research and design have succeeded in tailoring metals for a broad range of demands. CCAs are also crystalline materials but composed of three or more major elements in similar proportions.8 All major elements are principal ones. Consequently, they are chemically disordered corresponding to maximum configuration entropy, wherein the atom type randomly varies from one lattice site to another (Figure 1a). When actual CCAs are processed, homogenized, or annealed at temperatures well below their melting points, their chemical ordering deviates from being uniformly random. Chemical short-range order (CSRO) to medium-range order (i.e., local chemical order) inevitably appears in the system because of the attractive/repulsive interactions among the constituent elements (Figure 1b).
Portion of a polycrystalline equi-atomic CoCrNi. The mix of constituent elements at atomic sites are shown on the right and separately below. (a) Random solid solution and (b) chemical short-range ordering.7
The inventors originally referred to them as high-entropy alloys (HEAs), although over the years, HEAs have been variously called CCAs, compositionally complex alloys, multi-principal element alloys, medium-entropy alloys (MEAs, for those containing three majority elements), and multicomponent alloys.8,9,10 The motivation for “complex concentrated alloys” instead of H/MEAs is to acknowledge the complexity associated with these alloys, which involves chemical fluctuations, non-equimolar concentrations, and chemical short-range ordering, all within the vast compositional space.
Similar to their atomic constitution, CCAs exhibit unusual properties, such as extraordinary fracture toughness and a high-temperature strength plateau. CCAs composed of concentrated combinations of certain 3d transition-metal elements (e.g., Cr, Mn, Fe, Co, Ni, Cu) exhibit ultrahigh strengths exceeding those of most traditional alloys.11 A recent 2022 MRS Bulletin issue was dedicated to highlighting the superior mechanical and structural properties of high-entropy alloys and oxides.9,10,12 As a shining example, the equi-atomic, MEA, CoCrNi not only possess exceptionally high room-temperature fracture toughness, but its toughness increases when reduced to cryogenic temperatures.13 The strengths of refractory CCAs are easily three times higher strengths than that of any refractory constituent element and 50–300% the strength of leading superalloys.14 Some CCAs have shown much greater thermal stability and radiation resistance than pure or dilute-solute alloys.15,16,17
With strength as the main attraction of CCAs, a considerable body of basic research has been devoted to understanding the origins of their superior strength, so that they can be tailored for current and future needs. Studies on the strength of metallic crystals begin with knowledge of their atomic lattice structure. In this respect, conventional alloys and CCAs are alike; most possess one of two simple cubic structures, either face-centered cubic (fcc) or body-centered cubic (bcc). Such crystallographic information enables precise definition of the structure of two of the most influential crystalline imperfections impacting strength: dislocations and interstitials.18 Although the atomic fractions of these atomic “defects” are usually minute, their effect on strength is profound. Understanding strength relies on theories or models for the energies and mechanisms associated with mechanically driven defect formation and motion through the perfect crystalline lattice. Such atomic-scale descriptors of defects are founded on chemical periodicity and translational and rotational symmetries of the principal element. In this respect, CCAs diverge from pure metals and traditional alloys. From the atomic perspective, the constitutional and configurational randomness of the elements in the lattice in CCAs contradicts the classical assumptions of periodicity and symmetry. From the macroscopic viewpoint, this randomness can possibly explain the unusual strength properties of CCAs and redefine the way defects move.19,20,21 Further, any degree of CSRO in the CCA can affect such local defect processes, such as vacancy diffusion22 and dislocation motion. It has been some time since a new family of metal has stimulated a possible revision of the very basic foundations of structure, defects, and defect motion on which the field rests.
Articles in this issue are devoted to evaluating the latest tools and results produced for understanding CCAs “from the atom up,” the level at which CCAs possess their unique features. A special focus of many of the articles in this issue lies in CSRO. Methods presented include one or a combination of computational, theoretical, and experimental methods applied to gain new insights into local atomic structure and chemical ordering, defects, and unconventional phenomena that ensue. Most of the CCAs treated here are nominally fcc or bcc and single phase, not only because they serve as model systems of an otherwise complex material, but also because they represent the types receiving the most attention to date. Yet in the spirit of exploration and complexity, the systems reviewed here span from the non-equiatomic systems to ternary and quinary compositions to doped systems. From an assessment of recent results, each article offers next steps, possible redirections, or needs that can help meet the continual challenges in understanding single phase and even the more complex, multiphase CCAs.
CSRO
Earlier work treated CCAs as random solid solutions, where the probability of an element at a lattice site equals its mole fraction. However, the arrangement of atoms is not completely random due to local thermodynamically preferred bonding of certain atom types, resulting in CSRO23,24,25,26,27 (Figure 2a–b). CSRO treatments in CCAs to date are largely built upon those developed for binary alloys approximately 70 years ago. CSRO in binary alloys is usually measured by interpreting diffuse scattering of x-rays and is commonly quantified by probabilities of the like and unlike element pairings, which are used to define Warren–Cowley parameters.26,27 It has also been posited that CSRO plays a role in the properties of binaries, through its effect on dislocation glide stresses28 (Figure 2c–d). In recent years, CSRO in CCAs has been rapidly gaining attention. Compared to binaries, CSRO in CCAs can be much more difficult to theoretically describe, quantify, and computationally replicate, due to the increase in the number of possible pairings. For several fcc and bcc CCA systems, Warren–Cowley parameters have been adopted to describe CSRO in both experimental and computational studies. The article by Walsh et al.29 carefully examines the applicability of translating CSRO descriptors and methods, previously built for binaries, to CCAs. Drawing on examples of fcc CCAs, such as both equi- and non-equiatomic FeCoNiCu, FeCoNiCr, and FeCrMnNi, they evaluate the current understanding on the origin and role of CSRO and short-range clustering (SRC). For the benefit to future CCA research, they suggest alternative methods for description and measurement for CSRO and based on its ubiquity alone, argue that CSRO remains an important scientific area of study.
Cross section of elements in an equimolar complex concentrated alloy (CCA) ternary, containing three elements and exhibiting chemical short-range order. (a) Chemical fluctuations causing wavy glide of a dislocation. (b) Two constituent elements (red and blue) developing B2 order. (c) Screw-character dislocation gliding in a traditional alloy via kink-pair motion. (d) Same dislocation exhibiting wavy glide in a CCA. (c, d) Reprinted with permissions from Reference 17.
For CCAs, predicting CSRO preferred pairings and intensity has been enabled by atomistic simulation in combination with Monte Carlo (MC) methods.30,31 Such calculations start with an ideally random solid-solution atomic structure and proceed with a process of randomly swapping atoms according to an energy criterion under a finite (annealing) temperature until equilibrium is achieved. In a relatively rapid manner, such hybrid atomistic-MC methods can provide estimates of the extent and correlation lengths expected for a given combination of nominal compositions and heat treatment. For most CCAs, greater levels of CSRO are reported with lower annealing temperatures, if they lie above a critical temperature for phase separation or long-range ordering.21,25,32,33
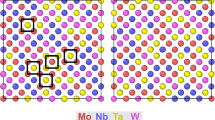
The article by Chen et al.34 highlights the latest computational techniques that have been deployed to determine CSRO in fcc and bcc CCAs. The methods reviewed range from density functional theory (DFT) to molecular dynamics (MD) simulations to statistical methods, such as cluster expansions (CEs) in combination with MC methods, and to machine learning (ML) methods. Valuably, the computational efficiency, expense, and validation involved are presented. For model systems, such as CoCrNi and MoNbTaW/V, they show that achieving first-principles-level accuracy for large systems, containing 1–3 M atoms, can be realized with use of ML interatomic potentials, using training data from DFT calculated bulk and defect properties. Experimental measurements of CSRO are reviewed and compared with results from computation for exemplar fcc CCAs, CoCrNi and CoCuFeNiPd. They contend that despite these successes, challenges in making CSRO/CCA–property linkages in these and similar systems persist and there is an unwanted dearth of similar studies in bcc and hcp CCAs. Further, considering a myriad of examples, they discuss the benefit of these computational techniques to identifying CSRO and its effects on phase stability.
With the departure from an ideal uniformly random structure, the question of the impact of CSRO on basic properties has emerged. CSRO changes the local atomic environments at the same fine scale where the structure and energetic barriers of strength-determining defects, such as dislocations and interstitials, are defined. With the atomic structures from MD/MC possessing equilibrium CSRO, subsequent calculations can be performed to assess the impact of CSRO on properties. The CSRO effect can be isolated by comparing outcomes starting with model structures with and without CSRO. Deploying this approach, most computational studies report nonnegligible consequences on stacking-fault energies, dislocation nucleation and mobility, deformation twinning, strength, and strain hardening.32,33,35
Studies by both experimental and computational approaches have suggested that CSRO could present a new dimension for tuning material functionalities. While this is ultimately the goal, such a strategy in part benefits from characterization. However, the fine nanometer length scales at which CSRO is expected to exist make direct measurement of CSRO features challenging. Modern tools of characterization have recently been employed to detect and identify CSRO as discussed in the article by Taheri et al.36 Some CCAs and processing conditions hint that the nature of CSRO in CCAs could be more intricate than first thought. CSRO could in fact appear as a heterogeneous set of local structures, including clusters (delineated by a discernible boundary) or nanostructures with an identifiable long-range order. Taheri et al. review examples that use advanced techniques spectroscopy, diffraction, and imaging to quantitatively measure and map CSRO in representative MoNbTaVW and MnFeCoNiCu, as well as Al-containing CCAs. They highlight the role of CSRO on the mechanisms of plasticity and high-temperature properties, as well as electrochemical behavior and other functional properties. Considering the prospects of more CSRO-dependent phenomena, the opportunities in CSRO-based alloy design are presented.
Interstitials
Interstitial elements such as carbon, nitrogen, and oxygen have varying solubility in transition metals. For example, oxygen solubility across transition-metal groups IVB to VIB reaches levels up to ~17 at.% in group VB, while being largely insoluble in group VIB elements. If solubility is low, then oxides, carbides or nitrides could be present, while high levels of interstitial solubility could alter phase transformation paths. The interstitial elements (often considered as impurities) have long been used to strengthen metals, for instance, carbon steel. This interstitial strengthening effect in pure bcc and fcc metals was interpreted and conceptualized by Cottrell and Bilby,37 known as Cottrell atmosphere. The segregation of carbon atoms around dislocations forms atmospheres, which relax dislocation core energies and increase dislocation glide barriers (dragging effect). Concerning CCAs, interstitials play an important role as seen through their influence on strength–ductility synergy, phase equilibria, and diffusional behaviors. For example, by adjusting the composition and oxygen content in a bcc TiZrNb, the phase stability and degree of oxygen complexes can be tuned to simultaneously improve strength and ductility.38 The article by Baker et al.39 provides a review of N and C interstitial effects on the mechanical and diffusional properties of fcc CoCrFeMnNi CCAs and its non-equiatomic variants. Specifically, the solubility of different interstitials and work-hardening rates, dislocation slip modes, and diffusion properties are discussed by analyzing and comparing recent experimental and computational results. In light of the important potential of bcc CCAs TiVZrNbHf and TiZrNbMoHf for hydrogen storage, the authors end with current findings on interstitial H in CCAs and recommendations for future work.
Dislocations
Dislocations, the line defects in crystal lattices, dictate the strength and deformation behavior of materials. Dislocation glide accommodates mechanical stress and results in microstructure evolution. To glide, they must overcome an energy barrier associated with lattice resistance. In pure metals, the energy landscape that governs dislocation motion is smooth. In contrast, in CCAs locally high chemical fluctuations inevitably change dislocation core energies and increase the “ruggedness” of the energy barrier landscape for dislocation motion.40 The fluctuating landscape translates to an extreme variability in the local slip resistances (LSRs), the minimum stress to translate a dislocation segment one lattice parameter, among distinct locations in the CCA crystal.40,41,42 The LSRs are greater than the Peierls stress of any constituent element and thus, we find that this fundamental quantity alone can explain why CCAs are stronger than their elements, the so-called “cocktail effect.”41,42 Yet, basic questions remain as to how rough the energy landscape is and to what extent it can be influenced by CSRO. By constructing the potential energy landscape governing screw dislocation motion in bcc MoNbTaW CCAs, a recent study revealed a hierarchical and multilevel structure with a collection of small basins nested in a large metabasin.40 This striking feature originates from the large chemical variation and exerts a trapping force and back stress on saddle point activations, retarding dislocation movement and providing an extra strengthening mechanism. CSRO was found to smooth this landscape from that for an ideal random solid solution.
The rugged energy landscape over which dislocations move strongly impacts the mechanisms of glide and consequently how a CCA plastically deforms. The article by Marian et al.43 discusses the recent advances in modeling dislocation glide processes and recent findings on the odd ways dislocations move in CCAs (Figure 2d). For a high-temperature bcc CCA MoNbTaW, MD simulations have revealed a cross-kinking to cross-kink annihilation transition in the glide of a screw dislocation as the temperature is raised from room temperature to 900 K. This knowledge can potentially explain why bcc CCAs sustain much higher strength at higher temperatures than any of their refractory constituents. The effects of chemical fluctuations on the motion of long dislocations and the resulting mesoscopic plastic response are captured best by multiscale modeling approaches, including those that link atomistic information and ML potentials to kinetic MC simulations or to phase field dislocation dynamics (PFDD) models. By treating longer dislocations over longer time scales than MD alone, these multiscale computations can reveal the mechanisms of glide and how they differ from those in conventional alloys.32 In many prototypical bcc CCAs, such as MoNbTaW, TaNbTi, and MoNbTi, both initially edge- and screw-oriented dislocations show wavy glide, glide plane hardening, and jerky motion, to a level not seen when in traditional alloys. With the ability to treat both ideally random and CSRO CCAs, PFDD models show that even relatively small degrees of CSRO can alter these glide dynamics. The authors envision a pathway to developing an integrated simulation framework that links composition-governed atomic-level mechanisms with mesoscopic mechanical responses to guide the design of CCAs for superior behavior.
When the CSRO is present in fcc CCAs, dislocation glide can destroy the local chemical ordering on the slip plane and create a diffuse antiphase boundary (APB). As APB generation incurs an energy penalty, the presence of CSRO would increase the stacking-fault energy associated with the APB and the barrier to dislocation glide. Alterations in the dislocation slip pathway and, hence, the deformation microstructures would ensue. Using ultra-large atomistic simulations, dislocation motion and patterning and deformation microstructure evolution in the fcc CrCoNi alloy have been examined.7,33 CSRO and the high SFE and APB generation resulting from its interaction with dislocation glide were shown to influence differently many key deformation processes, including dislocation slip, faulting, twinning, and phase transformation. With CSRO, faulting and deformation transformation are suppressed in CoCrNi, and planar slip is enhanced due to glide plane softening and repetitive slip. The CSRO-biased deformation processes discourage martensitic transformation and encourage strain localization.
Conclusions and outlook
Research over the past two decades have proven that CCAs are rapidly rising as an important metallic material family. The demands of future energy systems require radical improvements in the properties of metals, and CCAs will undoubtedly be a part of the solution. The alloy design space for CCAs is enormous, and only a small portion of this unlimited compositional space (mainly equimolar concentration) has been studied. Improving our ability to control material response with proper choice of composition, including non-equiatomic compositions, and thermal treatments will hasten their use in application. This leaves an enormous space to navigate and for this purpose, ML and artificial intelligence (AI) methods are being proposed and integrated for efficient exploration of compositional space and prediction of properties.44 In Figure 3, we demonstrate a perspective that combines defect calculations and ML to effectively explore the complete compositional dimension of CCAs and design alloys based on fundamental mechanisms. For example, ML-enabled composition space modeling has revealed a non-equiatomic NbMoTa composition exhibiting a higher diffusion barrier than its equimolar counterpart,45 implying that extraordinary behavior is not necessarily found in equimolar concentration but hidden in the huge non-equimolar space.
Although ML and AI are undoubtedly powerful tools, as part of the grand strategy, however, improving scientific understanding of CCAs at the fundamental levels remains a preeminent pursuit. Due to radical divergence in CCA constitution at an atomic scale from traditional alloys, the applicability of conventional theories, tools, and descriptors needs reconsideration and the utility of the most advanced, state-of-the-art techniques to date needs inclusion. Drawing on recent work, the articles in this issue tackle both fronts. They each include both experimental and modeling investigations that uncover essential processes determining the structure, kinetics, mechanisms, and properties in CCAs at the atomic scale.
Harnessing vast compositional space via machine learning (ML) and mechanism-based alloy design. ML models, that accurately capture the intricate chemical complexity and establish connections with defect properties such as vacancy diffusion and dislocation barrier,45 can efficiently explore the vast compositional space of complex concentrated alloys. Through complete sampling of the entire composition dimension, mechanism-based micro- and mesoscale models are developed for alloy design. The micro/mesoscale models include kinetic Monte Carlo (kMC), phase-field dislocation dynamics, and the crystal plasticity finite element method (CPFEM).
Looking forward, building on knowledge of atomic-scale phenomena, future CCA studies would benefit from examining a broader range of length scales, that is, from the atom up to microns. On the experimental side, while characterization at a fine, nanometer scale is valuable, local ordering phenomena in CCAs can possibly exist at multiple scales encouraging deployment of multiple characterization tools along with theory. Strategies to treat such heterogeneity would help meet additional challenges in characterizing multiphase or doped CCAs. On the computational side, atomistic calculations have shown that energy barriers for moving defects in CCAs statistically vary spatially and temporally. The statistical nature in the properties alone calls for performing large representative sizes and repeated instantiations. System sizes from atomistic simulations are potentially too small for calculating properties, such as strength and fracture toughness, which involve several dislocations and other mobile defects. Integrating multiple length scale modeling tools would enable linking small-scale effects to higher length scales at which responses can be measured. An example is the development of the OTIS code to quickly generate multiple realizations of large-sized CCA lattices with specified CSRO and energetic barriers from DFT or molecular statics calculations.46 Reflecting on the knowledge of today, the authors of the articles in this issue hope to inspire and accelerate basic studies that will underpin the design of new classes of materials in the vast compositional space waiting to be explored.
Data availability
All data generated or analyzed during this study are included in this published article.
References
J.-W. Yeh, S.K. Chen, S.J. Lin, J.Y. Gan, T.S. Chin, T.T. Shun, C.-H. Tsau, S.-Y. Chang, Adv. Eng. Mater. 6, 299 (2004)
B. Cantor, I.T.H. Chang, P. Knight, A.J.B. Vincent, Mater. Sci. Eng. A 375–377, 213 (2004)
O.N. Senkov, G. Wilks, D. Miracle, C. Chuang, P. Liaw, Intermetallics 18, 1758 (2010)
D.B. Miracle, O.N. Senkov, Acta Mater. 122, 448 (2017)
G.P. Easo, D. Raabe, R.O. Ritchie, Nat. Rev. Mater. 4, 515 (2019)
Z. Li, S. Zhao, R.O. Ritchie, M.A. Meyers, Prog. Mater. Sci. 102, 296 (2019)
P. Cao, Sci. Adv. 8, eabq7433 (2022)
B. Cantor, Prog. Mater. Sci. 120, 100754 (2021)
E.P. George, R.O. Ritchie, MRS Bull. 47(2), 145 (2022)
B. Gludovatz, R.O. Ritchie, MRS Bull. 47(2), 176 (2022)
B. Gludovatz, A. Hohenwarter, D. Catoor, E.H. Chang, E.P. George, R.O. Ritchie, Science 345, 1153 (2014)
W.A. Curtin, S.I. Rao, C. Woodward, MRS Bull. 47(2), 151 (2022)
B. Gludovatz, A. Hohenwarter, K.V. Thurston, H. Bei, Z. Wu, E.P. George, R.O. Ritchie, Nat. Commun. 7, 10602 (2016)
O.N. Senkov, D. Isheim, D.N. Seidman, A.L. Pilchak, Entropy (Basel) 18, 102 (2016)
F. Granberg, K. Nordlund, M.W. Ullah, K. Jin, C. Lu, H. Bei, L.M. Wang, F. Djurabekova, W.J. Weber, Y. Zhang, Phys. Rev. Lett. 116, 135504 (2016)
O.N. Senkov, S. Gorsse, D.B. Miracle, Acta Mater. 175, 394 (2019)
J.P. Couzinié, O.N. Senkov, D.B. Miracle, G. Dirras, Data Brief 21, 1622 (2018)
W. Cai, W.D. Nix, Imperfections in Crystalline Solids (Cambridge University Press, Cambridge, 2016)
E. Ma, Scr. Mater. 181, 127 (2020)
F. Wang, G.H. Balbus, X. Shuozhi, S. Yanqing, J. Shin, P.F. Rottmann, K.E. Knipling, J.-C. Stinville, L.H. Mills, O.N. Senkov, I.J. Beyerlein, T.M. Pollock, D.S. Gianola, Science 370, 95 (2020)
S. Xu, W.-R. Jian, I.J. Beyerlein, APL Mater. 10, 111107 (2022)
B. Xing, X. Wang, W.J. Bowman, P. Cao, Scr. Mater. 210, 114450 (2022)
F.X. Zhang, S. Zhao, K. Jin, H. Xue, G. Velisa, H. Bei, R. Huang, J.Y.P. Ko, D.C. Pagan, J.C. Neuefeind, W.J. Weber, Y. Zhang, Phys. Rev. Lett. 118, 205501 (2017)
R. Zhang, S. Zhao, J. Ding, Y. Chong, T. Jia, C. Ophus, M. Asta, R.O. Ritchie, A.M. Minor, Nature 581, 283 (2020)
S. Zhao, J. Phase Equilib. Diffus. 42, 578 (2021)
J.M. Cowley, Phys. Rev. 77, 669 (1950)
J.M. Cowley, Phys. Rev. 120, 1648 (1960)
J.C. Fisher, Acta Metall. 2, 9 (1954)
F. Walsh, A. Abu-Odeh, M. Asta, MRS Bull. 48(7), (2023). https://doi.org/10.1557/s43577-023-00555-y
M. Widom, W.P. Huhn, S. Maiti, W. Steurer, Metall. Mater. Trans. A 45, 196 (2014)
A. Fernández-Caballero, J.S. Wróbel, P.M. Mummery, D. Nguyen-Manh, J. Phase Equilib. Diffus. 38, 391 (2017)
H. Zheng, L.T.W. Fey, X.-G. Li, Y.-J. Hu, L. Qi, C. Chen, S. Xu, I.J. Beyerlein, S. Ping Ong, NPJ Comput. Mater. 9, 89 (2023)
Z. Xie, W.-R. Jian, S. Xu, I.J. Beyerlein, X. Zhang, Z. Wang, X. Yao, Acta Mater. 221, 117380 (2021)
W. Chen, L. Li, Q. Zhu, H. Zhuang, MRS Bull. 48(7), (2023). https://doi.org/10.1557/s43577-023-00575-8
J. Ding, Q. Yu, M. Asta, R.O. Ritchie, Proc. Natl Acad. Sci. U.S.A. 115, 8919 (2018)
M.L. Taheri, E. Anber, A. Barnett, S. Billinge, N. Birbilis, B. DeCost, D.L. Foley, E. Holcombe, J. Hollenbach, H. Joress, G. Leigh, Y. Rakita, J.M. Rondinelli, N. Smith, M.J. Waters, C. Wolverton, MRS Bull. (2023). https://doi.org/10.1557/s43577-023-00591-8
A.H. Cottrell, B.A. Bilby, Proc. Phys. Soc. A 62, 49 (1949)
M. Jiao, Z. Lei, Y. Wu, J. Du, X.-Y. Zhou, W. Li, X. Yuan, X. Liu, X. Zhu, S. Wang, H. Zhu, P. Cao, X. Liu, X. Zhang, H. Wang, S. Jiang, Z. Lu, Nat. Commun. 14, 806 (2023)
I. Baker, B. Grabowski, S.V. Divinski, X. Zhang, Y. Ikeda, MRS Bull. 48(7), (2023). https://doi.org/10.1557/s43577-023-00558-9
X. Wang, F. Maresca, P. Cao, Acta Mater. 234, 118022 (2022)
S. Xu, Y. Su, W.-R. Jian, I.J. Beyerlein, Acta Mater. 202, 68–79 (2021). https://doi.org/10.1016/j.actamat.2020.10.042
R.A. Romero, S. Xu, W.-R. Jian, I.J. Beyerlein, C.V. Ramana, Int. J. Plast. 149, 103157 (2022)
X. Zhou, X. Wang, L. Fey, S. He, I. Beyerlein, P. Cao, J. Marian, MRS Bull. 48(7), (2023). https://doi.org/10.1557/s43577-023-00571-y
C.K.H. Borg, C. Frey, J. Moh, T.M. Pollock, S. Gorsse, D.B. Miracle, O.N. Senkov, B. Meredig, J.E. Saal, Sci. Data 7, 430 (2020)
B. Xing, T.J. Rupert, X. Pan, P. Cao, Neural network kinetics: Diffusion multiplicity and B2 ordering in compositionally complex alloys (2023), Preprint, arXiv.2304.02957
L.T.W. Fey, I.J. Beyerlein, Integr. Mater. Manuf. Innov. 11, 382 (2022)
Acknowledgments
I.J.B. and T.M.P. gratefully acknowledge support from the Office of Naval Research under Contract ONR Grants N00014-21-1-2536 and N00014-22-1-2087, respectively. P.C. gratefully acknowledges support from the US Department of Energy (DOE), Office of Basic Energy Sciences, under Award No. DE-SC0022295.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Guest Editors state that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beyerlein, I.J., Cao, P. & Pollock, T.M. Complex concentrated alloys and chemical short-range ordering. MRS Bulletin 48, 746–752 (2023). https://doi.org/10.1557/s43577-023-00567-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-023-00567-8