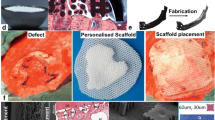
Abstract
Three-dimensional (3D) printing of scaffolds for tissue engineering applications has grown substantially in the past two decades. Unlike conventional autografts and allografts, 3D-printed scaffolds can satisfy the growing need for personalized bony reconstruction following massive craniofacial bone loss. Employing layer-by-layer manufacturing techniques, it is possible to produce patient-specific structures to rebuild complicated geometries for esthetic purposes and restore mechanical and respiratory functions. Here, we summarize the trends and current state-of-the-art studies in 3D-printing technologies for craniofacial bone reconstruction. We describe the design and development of the craniofacial scaffolds, including material choices, scaffold fabrication workflows, and the mechanical, structural, and biological considerations impacting scaffold application and function. Finally, we summarize the remaining hurdles and opportunities for growth to transition to the widespread clinical adoption of this technology.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Trends in three-dimensional printing for facial bone reconstruction
Why use three-dimensional printed scaffolds to regenerate facial bones?
Congenital abnormalities, trauma, or surgical excision of orofacial cancers are the most common etiologies of critical-sized craniofacial bone loss (i.e., bone injuries that, unlike simple fractures, will not heal spontaneously and without surgical intervention).1 These bony defects in the head and facial regions may impair patients’ vision, mastication, facial expression, and respiration and cause social and psychological stress. Currently, surgical reconstruction relies on the transplantation of bone from another part of the body into the defect in a process known as autologous bone grafting.2,3,4,5 However, drawbacks of this approach include the limited supply of donor tissue, donor site morbidity, complex and challenging surgeries, and high resorption rates, especially in pediatric patients.6 Alternatively, tissue engineering provides a promising strategy to facilitate tissue repair by transplanting cells and biomolecules into biomaterial scaffolds. To promote regeneration, the biomaterial scaffolds are designed with a high degree of interconnected porous structures to allow for vascular and bony ingrowth. Compared to biomaterials used to treat long bone defects, craniofacial scaffolds are characterized by their intricate morphologies and complex mechanical loading profiles that include periodic bending along with compressive and tensile forces. Consequently, designs based on medical imaging technology—usually cone-beam computed tomography (CBCT) and magnetic resonance imaging (MRI)—are used to recapitulate the anatomical shape of the missing bone and guide scaffold manufacture. Over the last two decades, three-dimensional (3D) printing methods have been increasingly applied to produce patient-matched devices, including instrumentation, implants, and external prostheses.7 Biomaterial fabrication using 3D printing for bone reconstruction (bone scaffolds) with complex anatomic shapes, controlled porous geometries, and bioactive properties to induce and guide new bone growth, is one of the fastest growing fields together with the advancement of 3D-printing techniques.8
Three-dimensional-printing technologies
The concept of 3D printing to fabricate objects by depositing materials layer by layer was first described by Hull in 19849 using a laser-based (stereolithography, SLA) technique and was subsequently applied to craniofacial surgery planning10,11 to provide surgeons with physiological models. Since then, laser-based printing methods have been used to print customized hydroxyapatite and titanium scaffolds to treat cranial and mandibular defects in clinical trials.12,13,14 Powder bed fusion uses laser or heat to fuse powders selectively with high print accuracy and resolution and can process a variety of powdered materials, including metals, bioceramics, and synthetic polymers. Material extrusion mechanically deposits semi-solid or molten filament in sequential layers to create 3D objects. Fused deposition modeling (FDM) is the most common form of material extrusion printing method, and it utilizes thermoplastic filaments as extrusion material. FDM can successfully recapitulate complex anatomies and produce highly regular pores15 but is not well suited to providing complex pore geometries. Polycaprolactone (PCL) scaffolds fabricated using FDM have been evaluated16,17 and applied in cranioplasties18 as burr hole plugs for the closure of post-trephination defects. Material jetting is a noncontact printing method that has been widely used to print multi-material and functionally graded materials. The printable ink of material jetting is limited to low viscosity ink. As a result, scaffolds fabricated by material jetting are usually accompanied by low mechanical strength and are not good candidates for load-bearing applications. The process of incorporating one or multiple types of living cells, biological molecules, and biomaterials to form scaffolds of desired 3D structure is called 3D bioprinting. Laser powder bed fusion, material extrusion, and material jetting all have been applied in bioprinting to produce a wide range of soft and hard tissues19,20,21 while avoiding high temperatures or organic solvents to maximize cell viability. For example, functional cartilage constructs with well-controlled complex architecture and improved mechanical strength were generated using hybrid inkjet printing/electrospinning system22 and cell-laden bioinks were directly deposited in situ using extrusion bioprinters to form multi-layer structures resembling natural skin.23
Advances in 3D-printed scaffolds for craniofacial bone regeneration
Three-dimensional-printing methods have been used to generate anatomically shaped scaffolds for use in craniofacial bone reconstruction for over two decades.24 As the technologies have advanced, so have the geometric complexities of the scaffolds (Figure 1). For example, Hollister et al. designed anatomic orbital floor structures from CT and MRI data25 by selecting the defect outline and filling the interior regions with porous architectures (Figure 1a). They further improved the design process to integrate a sleeve structure to enable the fixation of condylar-ramus scaffold to the adjacent native bone (Figure 1b).26 More recently, FDM methods have been used to print porous PCL maxillary scaffolds (Figure 1c)27 and mandibular scaffolds combining synthetic polymer and osteoinductive hydroxyapatite (Figure 1d)28 having more complex anatomic structures. Although scaffolds consisting of uniform pores are commonly used for their ease of design and fabrication, the actual mechanical property of craniofacial bone is nonhomogeneous. To better account for this in the designs, topology optimization29 may be used to design scaffolds with heterogeneous porous structures to vary the regional stiffness30,31,32 as shown in the orbital midface complex scaffold (Figure 1e).
(a) An orbital floor scaffold designed by substituting a porous structure into user selected craniofacial defect outline and printed from epoxy.25 (b) A polyurethane condylar-ramus unit, with a sleeve consisting of simple straight lines as border. The sleeve is designed to incorporate fixation screws (sagittal and coronal view).26 (c) Anatomically shaped polycaprolactone (PCL) maxilla scaffolds with consistent and regular pores, based on 3D models reconstructed from CT scan.27 (d) Anatomically shaped human mandible, comprising > 250 layers, is designed and 3D printed from hydroxyapatite-poly (lactic-co-glycolic acid) (HAPLGA) using solvent-based method at room temperature.28 (e) Anatomically shaped orbital midface complex scaffold with nonhomogeneous porosity, printed from acrylonitrile butadiene styrene (ABS) plastic filament. Regions with thin bones are assigned less porosity for stability, and thicker regions are assigned more porosity.32
Biomaterials used to 3D print craniofacial scaffolds
Materials selected for engineering bone scaffolds are biocompatible (able to support normal cellular activity without toxic effects to host tissue), bioresorbable (able to degrade at controlled rates while facilitating bone ingrowth), and mechanically strong (capable of withstanding mechanical loads during daily activity). For maxillomandibular scaffolds, there are unique requirements to withstand the periodic masticatory forces and accompanying bending, while for non-load-bearing defects (e.g., in the cranial region), the hardness, rather than the stiffness of the scaffold, may be more critical.
Bioceramics are popular material candidates for craniofacial bone regeneration, such as dental implants, alveolar ridge augmentation, and maxillofacial surgery, due to their wear resistance and similarities in composition to the inorganic matrix of bone. Hydroxyapatite (HA) is the predominant composition of bone matrix and possesses biocompatible and osteoconductive properties. HA has slow resorption rates, approximately ~ 1–2% per year in vivo,33 which limits its application in tissue engineering. Tricalcium phosphate (TCP), mainly β-TCP, has similar chemistry and structure to inorganic components in bone and forms a hydroxyapatite layer in body fluids. Bioactive glasses (BGs) possess good interfacial bond properties with bone34 and angiogenic properties in the presence of vascular endothelial growth factor (VEGF).35 Compared to bioceramics, the dissolution of soluble ions (such as silica and calcium) in BGs stimulate deposition of bone matrix by osteogenic cells and promotes faster and better integration between the scaffold and surrounding native bone tissues.36 However, bioceramics and BGs are relatively brittle and prone to failure under cyclic loading.37 Natural biopolymers, such as collagen and gelatin, have chemical compositions similar to natural bone organic matrix and are able to stimulate cell responses but natural polymers have weak compressive stiffnesses compared to native bone.38
Given the drawbacks of single-material scaffolds such as low biocompatibility and bioactivity of synthetic polymers38 and the unfavorable brittleness and degradation kinetics of bioceramics,33 there has been a trend toward composite scaffolds that combine different types of biomaterials (Figure 2). Different materials were directly combined via interlocking interfaces in earlier studies to form biphasic scaffolds (Figure 2a).39 More recently, composite scaffolds with uniform compositions, such as scaffolds combining BG and HA,40 BG and TCP,41,42 polypropylene (PP) and TCP,43 HA and PLGA or PCL (hyperelastic bone),44 and DCB particles and PCL (Figure 2b),45 have been 3D printed and many have been applied to critical-sized craniofacial defect treatment in vivo. PCL/HA mixtures with varying compositional and structural gradients have also been fabricated (Figure 2c).46 The ability to 3D print these composite materials with material extrusion methods enables the manufacture of craniofacial bone scaffolds with enhanced bioactivity.
Composite 3D-printed scaffolds for bone regeneration. (a) (Top) biphasic PLA/HA ceramic scaffold (top = PLA, blue = HA) fabricated from 3D-printed mode. PLA global pores are 600-μm diameter, HA global pores are 500 μm, (Bottom) colorized micro-CT of biphasic scaffold with portion cut away to depict interdigitation of both phases (yellow = PLA, blue = HA). Cut is at an angle to pore lattice to simultaneously view pore structure and material struts. Curved surfaces at polymer/ceramic interfaces are an artifact of image processing. PLA is in direct contact with HA.39 (b) Hybrid mandibular condyle scaffolds consisting of DCB particles and polycaprolactone (PCL), stained by Alizarin Red S to confirm and visualize the presence of mineralized particles in the hybrid scaffold.45 (c) Micro-CT images of dual-gradient PCL-HA scaffold, with HA concentration and porosity gradient in vertical direction (top: PCL and small pore of 0.2 mm, 85 wt% PCL, 15 wt% HA and medium pore of 0.5 mm, and 70 wt% PCL, 30 wt% HA, and large pore of 0.9 mm).46
Efficacy of 3D-printed scaffolds in craniofacial bone regeneration
Mechanical competence of 3D-printed craniofacial bone scaffolds
Ideally, craniofacial bone scaffolds would have mechanical strengths similar to that of natural bone. Stiffer scaffolds may promote “stress shielding” and result in bone resorption over time. Yet, it has typically proven more challenging to 3D print scaffolds with sufficient mechanical strength for load-bearing applications since the high porosity required to facilitate vascular, and cell invasion into the scaffolds during tissue regeneration requires a tradeoff with stiffness. Midfacial regions such as the nasal bone (most commonly seen midfacial fracture47) and buccal bone plate are geometrically complex and contain bone with regions that are less than 1 mm thick.48,49 Developing thin bioactive nasal scaffold with high anatomic fidelity is still challenging: In vivo studies using scaffolds printed from PCL resulted in vascular ingrowth after 2 months in rabbit nasal defects50 and after 3 months in porcine postauricular subcutaneous implantation.51 However, due to the tradeoffs required, the scaffolds either satisfied the thin structure of nasal bone but were printed in simple, rectangular shapes or they precisely replicated the nasal geometry but required thicker structures. Bone loss in the buccal bone plate is common after tooth extraction, and the resorption rate is higher for thinner plates.52 Unlike nasal bone, the buccal bone plates are constantly subjected to cyclic masticatory forces. Two distinct materials are used for treatment: synthetic PCL for the tooth replacement and metal meshes to repair the buccal plate. Synthetic PCL scaffolds have been printed into anatomic geometries and applied to fill the tooth socket following tooth extraction in pre-clinical studies and clinical trials.53,54 Due to mechanical strength requirement and volumetric limitations, only metal scaffolds such as titanium meshes are used to guide bone augmentation in the buccal plate.55
Bony integration of 3D-printed scaffolds
PCL is commonly used for 3D-printing bone scaffolds due to its biocompatibility, stability, and ease of standard fabrication. Many devices fabricated with PCL are approved by US Food and Drug Administration (FDA) approval for use in tissue engineering. However, it is not bioactive (i.e., on its own, it does not promote osteogenic differentiation). Thus, it is often used in conjunction with cells and growth factors (Figure 3). For example, by seeding bone morphogenetic protein-7 (BMP-7) transduced fibroblasts into pure PCL mandibular condyle scaffolds, new bone was formed following subcutaneous implantation in mice for 4 weeks (Figure 3a).56 Polyetherketoneketone (PEKK) is another good candidate for bone scaffolds and exhibits good biocompatibility and chemical stability. However, the poor integration with bone limits its application in bone reconstruction. Combining adipose-derived mesenchymal stem cells (ADSCs) can effectively enhance the bone regeneration capacity of 3D-printed PEKK scaffolds in treating mandibular defects (Figure 3b).57 To endow scaffolds with both angiogenic and osteogenic potential, dual delivery scaffolds, produced via cryogenic 3D printing of β-tricalcium phosphate (TCP)/osteogenic peptide (OP)/poly (lactic-co-glycolic) acid (PLGA) and coated with angiogenic peptide (AP)/collagen I hydrogel, were used to treat rat cranial defects.58 Angiogenesis induced by the quick release of AP and significantly improved new bone formation induced by sustained OP release were observed in vivo 3 months post-surgery (Figure 3c). Another strategy uses injectable scaffolds. Compared to the preformed 3D-printed scaffolds, injectable scaffolds are formed in vivo, minimally invasive, and can completely fill defects with irregular shapes with good margin adaptation and penetration depth. Since the first in vivo bioprinting was performed with nano-hydroxyapatite to treat mouse cranial defects,59 a wide range of hydrogels and polymers have been combined with multiple cell types and growth factors for dental and craniofacial tissue restoration in small animals.60,61 A 6-week in vivo study used injectable, UV-curable composite hydrogels with encapsulated periodontal ligament stem cells to treat rat alveolar bone defects and observed robust bone formation (Figure 3d).62
(a) Bone growth around PCL mandibular condyle orthogonal pore scaffold seeded with bone morphogenetic protein-7 (BMP-7) transduced fibroblasts (white: bone, blue: scaffold), after implanted subcutaneously in mice for 4 weeks.56 (b) Reconstructed micro-CT model showing bone ingrowth in a critical-sized defect in the mandible of a rabbit model, treated with PEKK/ADSCs composite, 20 weeks after transplantation (dashed red areas indicate the vertical bone ingrowth inside the PEKK scaffold).57 (c) Regenerated bone in rat cranial defect after 3 months transplantation of scaffold produced via cryogenic 3D printing of β-tricalcium phosphate (TCP) and osteogenic peptide (OP) containing water/poly(lactic-co-glycolic acid) (PLGA)/dichloromethane emulsion inks with angiogenic peptide (AP)/collagen I hydrogel coating. Dimension of defect is 5 mm × 3 mm.58 (d) Representative 3D reconstructed micro-CT images (left), new bone formation in transverse section (top right) and in region of interest (bottom right) in rat alveolar bone defect, 6 weeks after treated with in vivo bioprinting, showing robust bone formation. Periodontal ligament stem cells are encapsulated in injectable, photo-cross-linkable composite hydrogels. Hydrogels were directly injected into alveolar defect and UV cured.62 Scale bar = 1 mm.
Toward clinical trials and commercialization
In summary, 3D printing in craniofacial defect reconstruction integrates multiple scientific disciplines. Despite the use of 3D-printed hydroxyapatite,14,63 titanium,12,13 and PCL53 scaffolds in clinical trials to treat craniofacial defects, there remain hurdles preventing their widespread adoption:
-
Bone regeneration Current 3D-printed scaffolds enhance new tissue growth but fall short of complete healing. To improve bone regeneration in critical-sized bone defect, novel bioactive cues may be incorporated. For example, scaffolds releasing oxygen64,65 and soy isoflavones,66 dual delivery scaffolds suppressing bone resorption and promoting bone formation simultaneously,67 and scaffolds containing magnesium nanoparticles and graphene oxide to modulate immune responses68 are being investigated. Theoretical models have been developed to simulate the complex interaction between mechanobiological signals, native bone, and scaffolds.69,70 Machine learning has also been applied to provide more predictability during bone regeneration.71 Future research will focus on deepening the understanding of tissue healing biology and material science strategies to enhance scaffold bioactivity and increase control and predictability over the spatial and temporal bone formation.
-
Soft tissues coverage Reconstruction of maxillomandibular defects involves restoring cartilage (zonal organization, heterogeneous extracellular matrix composition, cell density, and mechanical property gradients), muscle and dental structures, which are all technically challenging due to the difficulty of engineering complex interfaces within a single construct. For example, efforts have been made to generate cartilage-bone constructs in temporomandibular joint defects and tooth-bone constructs.72,73 However, it remains a significant challenge to recapitulate the thin interface that enables the transition in cellular compositions, matrix structures, and mechanical properties that facilitate the proper functioning of the two tissues separately and as a single osteochondral unit.74
-
Manufacturing and Regulatory The design control requirements to ensure defined quality practices and procedures have been outlined by the FDA. Manufacturing materials, machinery, and process parameters can significantly affect scaffold print quality. Current effort in this area focuses on implementing design control to standardize the process from the initial design to the final validation, which includes the development of print quality standards for one-off, custom-designed scaffolds.
-
Commercialization Today, the imaging and geometric design of biomaterials have become a commercialized service. Additionally, the growing market opportunity has attracted many companies to expand their product lines following the industry’s shift toward biomaterials with regenerative capabilities. This has resulted in several start-up acquisitions within the last 15 years. The future widespread adoption of 3D-printed technologies is contingent upon the successful outcomes of pilot clinical studies where the inherent challenge is for the grafts to outperform autografts. Previous clinical trials of tissue engineering strategies for craniofacial bone reconstruction in Europe (which did not use 3D-printed scaffolds) did not demonstrate therapeutic advantages over the standard-of-care. Multiple pre-clinical studies are aimed at de-risking the process and overcoming biological and practical hurdles.
Data availability
Not applicable.
References
M.E. Elsalanty, D.G. Genecov, Craniomaxillofac. Trauma Reconstr. 2, 125 (2009). https://doi.org/10.1055/s-0029-1215875
P. Warnke, I. Springer, P.J. Wiltfang, P.Y. Acil, P.H. Eufinger, M. Wehmöller, P. Russo, H. Bolte, E. Sherry, E. Behrens, P.H. Terheyden, Lancet 364, 766 (2004). https://doi.org/10.1016/S0140-6736(04)16935-3
J. Wiltfang, M. Rohnen, J.H. Egberts, U. Lützen, H. Wieker, Y. Açil, H. Naujokat, Tissue Eng. C 22, 740 (2016). https://doi.org/10.1089/ten.tec.2015.0501
H. Kokemueller, S. Spalthoff, M. Nolff, F. Tavassol, H. Essig, C. Stuehmer, K.H. Bormann, M. Rücker, N.C. Gellrich, Int. J. Oral Maxillofac. Surg. 39, 379 (2010). https://doi.org/10.1016/j.ijom.2010.01.010
K. Mesimäki, B. Lindroos, J. Törnwall, J. Mauno, C. Lindqvist, R. Kontio, S. Miettinen, R. Suuronen, Int. J. Oral Maxillofac. Surg. 38, 201 (2009). https://doi.org/10.1016/j.ijom.2009.01.001
G.A. Grant, M. Jolley, R.G. Ellenbogen, T.S. Roberts, J.R. Gruss, J.D. Loeser, J. Neurosurg. Pediatr. 100, 163 (2004). https://doi.org/10.3171/ped.2004.100.2.0163
S. Bose, D. Ke, H. Sahasrabudhe, A. Bandyopadhyay, Prog. Mater. Sci. 93, 45 (2018). https://doi.org/10.1016/J.PMATSCI.2017.08.003
S. Bose, S. Vahabzadeh, A. Bandyopadhyay, Mater. Today 16, 496 (2013). https://doi.org/10.1016/j.mattod.2013.11.017
C. Hull, US4575330A, 1984. https://patents.google.com/patent/US4575330A/en
J.S. Bill, J.F. Reuther, Mund Kiefer Gesichtschir. 8, 135 (2004). https://doi.org/10.1007/s10006-004-0541-0
N.J. Mankovich, A.M. Cheeseman, N.G. Stoker, J. Digit. Imaging 3, 200 (1990). https://doi.org/10.1007/BF03167610
T. Sumida, N. Otawa, Y. Kamata, S. Kamakura, T. Mtsushita, H. Kitagaki, S. Mori, K. Sasaki, S. Fujibayashi, M. Takemoto, A. Yamaguchi, T. Sohmura, T. Nakamura, Y. Mori, J. Cranio-Maxillofac. Surg. 43, 2183 (2015). https://doi.org/10.1016/j.jcms.2015.10.020
E.-K. Park, J.-Y. Lim, I.-S. Yun, J.-S. Kim, S.-H. Woo, D.-S. Kim, K.-W. Shim, J. Craniofac. Surg. 27, 943 (2016). https://doi.org/10.1097/SCS.0000000000002656
J. Brie, T. Chartier, C. Chaput, C. Delage, B. Pradeau, F. Caire, M.-P. Boncoeur, J.-J. Moreau, J. Cranio-Maxillofac. Surg. 41, 403 (2013). https://doi.org/10.1016/j.jcms.2012.11.005
E. Nyberg, A. Rindone, A. Dorafshar, W.L. Grayson, Tissue Eng. A 23, 503 (2017). https://doi.org/10.1089/ten.tea.2016.0418
D.W. Hutmacher, Biomaterials 21, 2529 (2000). https://doi.org/10.1016/S0142-9612(00)00121-6
I. Zein, D.W. Hutmacher, K.C. Tan, S.H. Teoh, Biomaterials 23, 1169 (2002). https://doi.org/10.1016/S0142-9612(01)00232-0
J.-T. Schantz, T.-C. Lim, C. Ning, S.H. Teoh, K.C. Tan, S.C. Wang, D.W. Hutmacher, Oper. Neurosurg. (2006). https://doi.org/10.1227/01.NEU.0000193533.54580.3F
A. Bandyopadhyay, S. Bose, S. Das, MRS Bull. 40, 108 (2015). https://doi.org/10.1557/mrs.2015.3
P. Rider, ŽP. Kačarević, S. Alkildani, S. Retnasingh, M. Barbeck, J. Tissue Eng. 9, 204173141880209 (2018). https://doi.org/10.1177/2041731418802090
A. Ovsianikov, M. Gruene, M. Pflaum, L. Koch, F. Maiorana, M. Wilhelmi, A. Haverich, B. Chichkov, Biofabrication 2, 014104 (2010). https://doi.org/10.1088/1758-5082/2/1/014104
T. Xu, K.W. Binder, M.Z. Albanna, D. Dice, W. Zhao, J.J. Yoo, A. Atala, Biofabrication 5, 015001 (2012). https://doi.org/10.1088/1758-5082/5/1/015001
M. Albanna, K.W. Binder, S.V. Murphy, J. Kim, S.A. Qasem, W. Zhao, J. Tan, I.B. El-Amin, D.D. Dice, J. Marco, J. Green, T. Xu, A. Skardal, J.H. Holmes, J.D. Jackson, A. Atala, J.J. Yoo, Sci. Rep. 9, 1856 (2019). https://doi.org/10.1038/s41598-018-38366-w
C. Wang, W. Huang, Y. Zhou, L. He, Z. He, Z. Chen, X. He, S. Tian, J. Liao, B. Lu, Y. Wei, M. Wang, Bioact. Mater. 5, 82 (2020). https://doi.org/10.1016/J.BIOACTMAT.2020.01.004
S.J. Hollister, R.A. Levy, T.-M. Chu, J.W. Halloran, S.E. Feinberg, Int. J. Oral Maxillofac. Surg. 29, 66 (2000). https://doi.org/10.1016/S0901-5027(00)80128-9
S.E. Feinberg, S.J. Hollister, J.W. Halloran, T.M.G. Chu, P.H. Krebsbach, Cells Tissues Organs 169, 309 (2001). https://doi.org/10.1159/000047896
J.P. Temple, D.L. Hutton, B.P. Hung, P.Y. Huri, C.A. Cook, R. Kondragunta, X. Jia, W.L. Grayson, J. Biomed. Mater. Res. A 102, 4317 (2014). https://doi.org/10.1002/JBM.A.35107
A.E. Jakus, A.L. Rutz, S.W. Jordan, A. Kannan, S.M. Mitchell, C. Yun, K.D. Koube, S.C. Yoo, H.E. Whiteley, C.P. Richter, R.D. Galiano, W.K. Hsu, S.R. Stock, E.L. Hsu, R.N. Shah, Sci. Transl. Med. (2016). https://doi.org/10.1126/scitranslmed.aaf7704
M.P. Bendsøe, N. Kikuchi, Comput. Methods Appl. Mech. Eng. 71, 197 (1988). https://doi.org/10.1016/0045-7825(88)90086-2
O. Sigmund, Int. J. Solids Struct. 31, 2313 (1994). https://doi.org/10.1016/0020-7683(94)90154-6
O. Sigmund, Philos. Trans. R. Soc. Lond. Ser. A 358, 211 (2000). https://doi.org/10.1098/rsta.2000.0528
E. Nyberg, A. O’Sullivan, W. Grayson, PLoS ONE (2019). https://doi.org/10.1371/journal.pone.0225007
W.R. Moore, S.E. Graves, G.I. Bain, ANZ J. Surg. 71, 354 (2001). https://doi.org/10.1046/J.1440-1622.2001.02128.X
L.L. Hench, R.J. Splinter, W.C. Allen, T.K. Greenlee, J. Biomed. Mater. Res. 5, 117 (1971). https://doi.org/10.1002/jbm.820050611
J. Kent Leach, D. Kaigler, Z. Wang, P.H. Krebsbach, D.J. Mooney, Biomaterials 27, 3249 (2006). https://doi.org/10.1016/J.BIOMATERIALS.2006.01.033
C. Vyas, G. Poologasundarampillai, J. Hoyland, P. Bartolo, Biomedical Composition (Elsevier, Amsterdam, 2017), p. 261. https://doi.org/10.1016/B978-0-08-100752-5.00013-5
J.R. Jones, Acta Biomater. 9, 4457 (2013). https://doi.org/10.1016/j.actbio.2012.08.023
M. Filippi, G. Born, M. Chaaban, A. Scherberich, Front. Bioeng. Biotechnol. 8, 474 (2020). https://doi.org/10.3389/fbioe.2020.00474
J.M. Taboas, R.D. Maddox, P.H. Krebsbach, S.J. Hollister, Biomaterials 24, 181 (2003). https://doi.org/10.1016/S0142-9612(02)00276-4
A. Winkel, R. Meszaros, S. Reinsch, R. Müller, N. Travitzky, T. Fey, P. Greil, L. Wondraczek, J. Am. Ceram. Soc. 95, 3387 (2012). https://doi.org/10.1111/J.1551-2916.2012.05368.X
C. Bergmann, M. Lindner, W. Zhang, K. Koczur, A. Kirsten, R. Telle, H. Fischer, J. Eur. Ceram. Soc. 30, 2563 (2010). https://doi.org/10.1016/J.JEURCERAMSOC.2010.04.037
S. Bose, A. Bhattacharjee, D. Banerjee, A.R. Boccaccini, A. Bandyopadhyay, Addit. Manuf. 40, 101895 (2021). https://doi.org/10.1016/J.ADDMA.2021.101895
S.J. Kalita, S. Bose, H.L. Hosick, A. Bandyopadhyay, Mater. Sci. Eng. C 23, 611 (2003). https://doi.org/10.1016/S0928-4931(03)00052-3
Y.H. Huang, A.E. Jakus, S.W. Jordan, Z. Dumanian, K. Parker, L. Zhao, P.K. Patel, R.N. Shah, Plast. Reconstr. Surg. 143, 1397 (2019). https://doi.org/10.1097/PRS.0000000000005530
B.P. Hung, B.A. Naved, E.L. Nyberg, M. Dias, C.A. Holmes, J.H. Elisseeff, A.H. Dorafshar, W.L. Grayson, ACS Biomater. Sci. Eng. (2016). https://doi.org/10.1021/acsbiomaterials.6b00101
S.M. Bittner, B.T. Smith, L. Diaz-Gomez, C.D. Hudgins, A.J. Melchiorri, D.W. Scott, J.P. Fisher, A.G. Mikos, Acta Biomater. 90, 37 (2019). https://doi.org/10.1016/J.ACTBIO.2019.03.041
K.-S. Jin, H. Lee, J.-B. Sohn, Y.-S. Han, D.-U. Jung, H.-Y. Sim, H.-S. Kim, Maxillofac. Plast. Reconstr. Surg. (2018). https://doi.org/10.1186/s40902-018-0168-y
M.J. Citardi, S. Hardeman, C. Hollenbeak, M. Kokoska, Arch. Otolaryngol. Neck Surg. 126, 979 (2000). https://doi.org/10.1001/ARCHOTOL.126.8.979
A.L. Januário, W.R. Duarte, M. Barriviera, J.C. Mesti, M.G. Araújo, J. Lindhe, Clin. Oral Implants Res. (2011). https://doi.org/10.1111/j.1600-0501.2010.02086.x
S.H. Park, B.G. Yun, J.Y. Won, W.S. Yun, J.H. Shim, M.H. Lim, D.H. Kim, S.A. Baek, Y.D. Alahmari, J.H. Jeun, S.H. Hwang, S.W. Kim, Laryngoscope. 127, 1036 (2017). https://doi.org/10.1002/LARY.26400
D.A. Zopf, A.G. Mitsak, C.L. Flanagan, M. Wheeler, G.E. Green, S.J. Hollister, Otolaryngol. Neck Surg. 152, 57 (2015). https://doi.org/10.1177/0194599814552065
M. Qahash, C. Susin, G. Polimeni, J. Hall, U.M. Wikesjo, Clin. Oral Implants Res. 19, 166 (2008). https://doi.org/10.1111/J.1600-0501.2007.01428.X
B.T. Goh, L.Y. Teh, D.B.P. Tan, Z. Zhang, S.H. Teoh, Clin. Oral Implants Res. 26, 271 (2015). https://doi.org/10.1111/clr.12486
J. Viña-Almunia, M.E. Candel-Martí, J. Cervera-Ballester, B. García-Mira, J.L. Calvo-Guirado, D. Peñarrocha-Oltra, M. Peñarrocha-Diago, Implant Dent. 22, 155 (2013). https://doi.org/10.1097/ID.0B013E318287A947
L. Ciocca, G. Lizio, P. Baldissara, A. Sambuco, R. Scotti, G. Corinaldesi, J. Oral Implantol. 44, 131 (2018). https://doi.org/10.1563/AAID-JOI-D-17-00125
J.M. Williams, A. Adewunmi, R.M. Schek, C.L. Flanagan, P.H. Krebsbach, S.E. Feinberg, S.J. Hollister, S. Das, Biomaterials 26, 4817 (2005). https://doi.org/10.1016/J.BIOMATERIALS.2004.11.057
M.G. Roskies, D. Fang, M.-N. Abdallah, A.M. Charbonneau, N. Cohen, J.O. Jordan, M.P. Hier, A. Mlynarek, F. Tamimi, S.D. Tran, Laryngoscope. 127, E392 (2017). https://doi.org/10.1002/lary.26781
C. Wang, J. Lai, K. Li, S. Zhu, B. Lu, J. Liu, Y. Tang, Y. Wei, Bioact. Mater. 6, 137 (2021). https://doi.org/10.1016/j.bioactmat.2020.07.007
V. Keriquel, F. Guillemot, I. Arnault, B. Guillotin, S. Miraux, J. Amédée, J.-C. Fricain, S. Catros, Biofabrication 2, 014101 (2010). https://doi.org/10.1088/1758-5082/2/1/014101
B. Chang, N. Ahuja, C. Ma, X. Liu, Mater. Sci. Eng. R 111, 1 (2017). https://doi.org/10.1016/j.mser.2016.11.001
P. Wang, Y. Song, M.D. Weir, J. Sun, L. Zhao, C.G. Simon, H.H.K. Xu, Dent. Mater. 32, 252 (2016). https://doi.org/10.1016/j.dental.2015.11.019
Y. Ma, Y. Ji, T. Zhong, W. Wan, Q. Yang, A. Li, X. Zhang, M. Lin, ACS Biomater. Sci. Eng. 3, 3534 (2017). https://doi.org/10.1021/acsbiomaterials.7b00601
H. Saijo, K. Igawa, Y. Kanno, Y. Mori, K. Kondo, K. Shimizu, S. Suzuki, D. Chikazu, M. Iino, M. Anzai, N. Sasaki, U. Chung, T. Takato, J. Artif. Organs 12, 200 (2009). https://doi.org/10.1007/s10047-009-0462-7
C.A. Cook, K.C. Hahn, J.B.F. Morrissette-McAlmon, W.L. Grayson, Biomaterials 52, 376 (2015). https://doi.org/10.1016/J.BIOMATERIALS.2015.02.036
M. Touri, F. Moztarzadeh, N.A. Abu Osman, M.M. Dehghan, P. Brouki Milan, S. Farzad-Mohajeri, M. Mozafari, ACS Biomater. Sci. Eng. 6, 2985 (2020). https://doi.org/10.1021/acsbiomaterials.9b01789
N. Sarkar, S. Bose, Acta Biomater. 114, 407 (2020). https://doi.org/10.1016/j.actbio.2020.07.006
Y. Wang, W. Cui, X. Zhao, S. Wen, Y. Sun, J. Han, H. Zhang, Nanoscale 11, 60 (2019). https://doi.org/10.1039/C8NR07329E
Z. Zheng, Y. Chen, H. Hong, Y. Shen, Y. Wang, J. Sun, X. Wang, Adv. Healthc. Mater. 10, 2000631 (2021). https://doi.org/10.1002/adhm.202000631
M.-I. Pastrama, S. Scheiner, P. Pivonka, C. Hellmich, Bone 107, 208 (2018). https://doi.org/10.1016/j.bone.2017.11.009
C. Perier-Metz, G.N. Duda, S. Checa, Front. Bioeng. Biotechnol. (2020). https://doi.org/10.3389/FBIOE.2020.585799
C. Wu, A. Entezari, K. Zheng, J. Fang, H. Zreiqat, G.P. Steven, M.V. Swain, Q. Li, Nat. Comput. Sci. (2021). https://doi.org/10.1038/s43588-021-00115-x
D. Chen, J.Y. Wu, K.M. Kennedy, K. Yeager, J.C. Bernhard, J.J. Ng, B.K. Zimmerman, S. Robinson, K.M. Durney, C. Shaeffer, O.F. Vila, C. Takawira, J.M. Gimble, X. Edward Guo, G.A. Ateshian, M.J. Lopez, S.B. Eisig, G. Vunjak-Novakovic, Sci. Transl. Med. (2020). https://doi.org/10.1126/scitranslmed.abb6683
H. Abukawa, W. Zhang, C.S. Young, R. Asrican, J.P. Vacanti, L.B. Kaban, M.J. Troulis, P.C. Yelick, J. Oral Maxillofac. Surg. 67, 335 (2009). https://doi.org/10.1016/j.joms.2008.09.002
E.C. Beck, M.S. Detamore, Nanomaterials in Tissue Engineering (Elsevier, Amsterdam, 2013), p. 363. https://doi.org/10.1533/9780857097231.3.363
Funding
This work was supported by National Institutes of Health Grant No. 5R01DE027957 (WLG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Grayson, W. Three-dimensional printing of scaffolds for facial reconstruction. MRS Bulletin 47, 91–97 (2022). https://doi.org/10.1557/s43577-021-00261-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-021-00261-7