Abstract
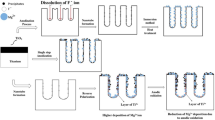
Surface modification of titanium and titanium alloys is a common method to improve anchoring of bone tissue and implants in hard tissue engineering applications. In the current work, a combination of chemical and physical methods (anodization and physical vapor deposition) was used to roughen the titanium surface and deposit iron (Fe) on the surface of titanium at different thicknesses. The optimized thickness of 100 Å was selected for mechanical and biological characterization. We found that anodization increases the surface roughness of Ti from 21 ± 0 to 229 ± 9 nm, whereas Fe deposition does not change it significantly. Our results also showed that surface modification of Ti by anodization increases the proliferation of osteosarcoma cells at both time points, whereas Fe-deposited samples showed the lowest cellular activity. These results suggest that Fe-deposited Ti implants may be suitable candidates for patients with osteosarcoma, as the proliferation of malignant cells decreases in the presence of Fe.






Similar content being viewed by others
References
G. Manivasagam, A.K. Singh, A. Rajamanickam, and A. Gogia: Ti based biomaterials, the ultimate choice for orthopaedic implants: A review. Prog. Mater. Sci. 54, 397–425 (2009).
F. Suska, L. Emanuelsson, A. Johansson, P. Tengvall, and P. Thomsen: Fibrous capsule formation around titanium and copper. J. Biomed. Mater. Res. 85, 888–896 (2008).
A. Jemat, M.J. Ghazali, M. Razali, and Y. Otsuka: Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 1–11 (2015).
M-A. Cha, C. Shin, D. Kannaiyan, Y.H. Jang, S.T. Kochuveedu, D.Y. Ryu, and D.H. Kim: A versatile approach to the fabrication of TiO2 nanostructures with reverse morphology and mesoporous Ag/TiO2 thin films via cooperative PS-b-PEO self-assembly and a sol–gel process. J. Mater. Chem. 19, 7245–7250 (2009).
W.A. Camargo, S. Takemoto, J.W. Hoekstra, S.C.G. Leeuwenburgh, J.A. Jansen, J.J.J.P. van den Beucken, and H.S. Alghamdi: Effect of surface alkali-based treatment of titanium implants on ability to promote in vitro mineralization and in vivo bone formation. Acta Biomater. 57, 511–523 (2017).
S. Jain, R. Scott Williamson, and M.D. Roach: Surface characterization, shear strength, and bioactivity of anodized titanium prepared in mixed-acid electrolytes. Surf. Coat. Technol. 325, 594–603 (2017).
Z.U. Rahman, I. Shabib, and W. Haider: Surface characterization and cytotoxicity analysis of plasma sprayed coatings on titanium alloys. Mater. Sci. Eng., C 67, 675–683 (2016).
F. Hempel, B. Finke, C. Zietz, R. Bader, K-D. Weltmann, and M. Polak: Antimicrobial surface modification of titanium substrates by means of plasma immersion ion implantation and deposition of copper. Surf. Coat. Technol. 256, 52–58 (2014).
K-Y. Hung, H-C. Lai, H-P. Feng, K-Y. Hung, H-C. Lai, and H-P. Feng: Characteristics of RF-sputtered thin films of calcium phosphate on titanium dental implants. Coatings 7, 126–135 (2017).
M. Roy, A. Bandyopadhyay, and S. Bose: Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Technol. 205, 2785–2792 (2011).
N.A. El-wassefy, I.M. Hammouda, A.N.E.A. Habib, G.Y. El-awady, and H.A. Marzook: Assessment of anodized titanium implants bioactivity. Clin. Oral Implants Res. 25, e1–e9 (2014).
Y. Zhang, R. Luo, J. Tan, J. Wang, X. Lu, S. Qu, J. Weng, and B. Feng: Osteoblast behaviors on titania nanotube and mesopore layers. Regen. Biomater. 4, 81–87 (2017).
L. Lv, Y. Liu, P. Zhang, X. Zhang, J. Liu, T. Chen, P. Su, H. Li, and Y. Zhou: The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 39, 193–205 (2015).
S. Oh, K.S. Brammer, Y.S.J. Li, D. Teng, A.J. Engler, S. Chien, and S. Jin: Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. 106, 2130–2135 (2009).
M.K. Duvvuru, W. Han, P.R. Chowdhury, S. Vahabzadeh, F. Sciammarella, and S.F. Elsawa: Bone marrow stromal cells interaction with titanium; Effects of composition and surface modification. PloS One 14, e0216087 (2019).
C. Liu, Y. Zhang, L. Wang, X. Zhang, Q. Chen, and B. Wu: A strontium-modified titanium surface produced by a new method and its biocompatibility in vitro. PloS One 10, e0140669 (2015).
Y. Liang, J. Xu, J. Chen, M. Qi, X. Xie, and M. Hu: Zinc ion implantation-deposition technique improves the osteoblast biocompatibility of titanium surfaces. Mol. Med. Rep. 11, 4225–4231 (2015).
R. Pokrowiecki, T. Zaręba, B. Szaraniec, K. Pałka, A. Mielczarek, E. Menaszek, and S. Tyski: In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 12, 42854297 (2017).
X. Li, Q. Huang, L. Liu, W. Zhu, T.A. Elkhooly, Y. Liu, Q. Feng, Q. Li, S. Zhou, Y. Liu, and H. Wu: Reduced inflammatory response by incorporating magnesium into porous TiO2 coating on titanium substrate. Colloids Surf., B 171, 276–284 (2018).
W. Liu, N.H. Golshan, X. Deng, D.J. Hickey, K. Zeimer, H. Li, and T.J. Webster: Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale 8, 15783–15794 (2016).
S. Vahabzadeh and S. Bose: Effects of iron on physical and mechanical properties, and osteoblast cell interaction in β-tricalcium phosphate. Ann. Biomed. Eng. 45, 819–828 (2017).
S. Du, J. Li, C. Du, Z. Huang, G. Chen, and W. Yan: Overendocytosis of superparamagnetic iron oxide particles increases apoptosis and triggers autophagic cell death in human osteosarcoma cell under a spinning magnetic field. Oncotarget 8, 9410–9424 (2016).
S.V. Torti and F.M. Torti: Iron and cancer: More ore to be mined. Nat. Rev. Cancer 13, 342–355 (2013).
S. Liu and Q.J. Wang: Determination of Young’s modulus and Poisson’s ratio for coatings. Surf. Coat. Technol. 201, 6470–6477 (2007).
S.A. Alves, A.R. Ribeiro, S. Gemini-Piperni, R.C. Silva, A.M. Saraiva, P.E. Leite, G. Perez, S.M. Oliveira, J.R. Araujo, B.S. Archanjo, M.E. Rodrigues, M. Henriques, J-P. Celis, T. Shokuhfar, R. Borojevic, J.M. Granjeiro, and L.A. Rocha: TiO2 nanotubes enriched with calcium, phosphorous and zinc: Promising bio-selective functional surfaces for osseointegrated titanium implants. RSC Adv. 7, 49720–49738 (2017).
K. Gulati, S. Ramakrishnan, M.S. Aw, G.J. Atkins, D.M. Findlay, and D. Losic: Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 8, 449–456 (2012).
V.B. Damodaran, D. Bhatnagar, V. Leszczak, and K.C. Popat: Titania nanostructures: A biomedical perspective. RSC Adv. 5, 37149–37171 (2015).
D.A. Tallarico, A.L. Gobbi, P.I. Paulin Filho, M.E.H. Maia da Costa, and P.A.P. Nascente: Growth and surface characterization of TiNbZr thin films deposited by magnetron sputtering for biomedical applications. Mater. Sci. Eng., C 43, 45–49 (2014).
M. Lilja, A. Genvad, M. Astrand, M. Strømme, and H. Enqvist: Influence of microstructure and chemical composition of sputter deposited TiO2 thin films on in vitro bioactivity. J. Mater. Sci. Mater. Med. 22, 2727–2734 (2011).
P.A. Savale: Physical vapor deposition (PVD) methods for synthesis of thin films: A comparative study. Arch. Appl. Sci. Res. 8, 1–8 (2016).
B. S Smith, S. Yoriya, L. Grissom, C. A Grimes, and K. Popat: Hemocompatibility of titania nanotube arrays. J. Biomed. Mater. Res., Part A 95A, 350–360 (2011).
K. Indira, U.K. Mudali, and N. Rajendran: In vitro biocompatibility and corrosion resistance of strontium incorporated TiO2 nanotube arrays for orthopaedic applications. J. Biomater. Appl. 29, 113–129 (2014).
K. Das, S. Bose, and A. Bandyopadhyay: TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res., Part A 90, 225–237 (2009).
S.A. Alves, A.L. Rossi, A.R. Ribeiro, F. Toptan, A.M. Pinto, J-P. Celis, T. Shokuhfar, and L.A. Rocha: Tribo-electrochemical behavior of bio-functionalized TiO2 nanotubes in artificial saliva: Understanding of degradation mechanisms. Wear 384–385, 28 (2017).
A. Hamlekhan, A. Butt, S. Patel, D. Royhman, C. Takoudis, C. Sukotjo, J. Yuan, G. Jursich, M.T. Mathew, W. Hendrickson, A. Virdi, and T. Shokuhfar: Fabrication of anti-aging TiO2 nanotubes on biomedical Ti alloys. PloS One 9, e96213 (2014).
S.A. Alves, A.L. Rossi, A.R. Ribeiro, F. Toptan, A.M. Pinto, T. Shokuhfar, J-P. Celis, and L.A. Rocha: Improved tribocorrosion performance of bio-functionalized TiO2 nanotubes under two-cycle sliding actions in artificial saliva. J. Mech. Behav. Biomed. Mater. 80, 143–154 (2018).
T. Shokuhfar, G.K. Arumugam, P.A. Heiden, R.S. Yassar, and C. Friedrich: Direct compressive measurements of individual titanium dioxide nanotubes. ACS Nano 3, 3098–3102 (2009).
G.A. Crawford, N. Chawla, and J.E. Houston: Nanomechanics of biocompatible TiO2 nanotubes by interfacial force microscopy (IFM). J. Mech. Behav. Biomed. Mater. 2, 580–587 (2009).
Y.N. Xu, M.N. Liu, M.C. Wang, A. Oloyede, J.M. Bell, and C. Yan: Nanoindentation study of the mechanical behavior of TiO2 nanotube arrays. J. Appl. Phys. 118, 145301 (2015).
H. Tang, Y. Li, J. Ma, X. Zhang, B. Li, S. Liu, F. Dai, and X. Zhang: Improvement of biological and mechanical properties of titanium surface by anodic oxidation. Bio-Med. Mater. Eng. 27, 485–494 (2016).
M-T. Tsai, Y-Y. Chang, H-L. Huang, Y-H. Wu, and T-M. Shieh: Micro-arc oxidation treatment enhanced the biological performance of human osteosarcoma cell line and human skin fibroblasts cultured on titanium–zirconium films. Surf. Coat. Technol. 303, 268–276 (2016).
A. Bandyopadhyay, A. Shivaram, S. Tarafder, H. Sahasrabudhe, D. Banerjee, and S. Bose: In vivo response of laser processed porous titanium implants for load-bearing implants. Ann. Biomed. Eng. 45, 249–260 (2017).
C. Yao, E.B. Slamovich, and T.J. Webster: Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res., Part A 85, 157–166 (2008).
A. Shivaram, S. Bose, and A. Bandyopadhyay: Mechanical degradation of TiO2 nanotubes with and without nanoparticulate silver coating. J. Mech. Behav. Biomed. Mater. 59, 508–518 (2016).
S.A. Alves, S.B. Patel, C. Sukotjo, M.T. Mathew, P.N. Filho, J-P. Celis, L.A. Rocha, and T. Shokuhfar: Synthesis of calcium-phosphorous doped TiO2 nanotubes by anodization and reverse polarization: A promising strategy for an efficient biofunctional implant surface. Appl. Surf. Sci. 399, 682–701 (2017).
L. Le Guéhennec, A. Soueidan, P. Layrolle, and Y. Amouriq: Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 23, 844–854 (2007).
C. Vasilescu, P. Drob, E. Vasilescu, I. Demetrescu, D. Ionita, M. Prodana, and S.I. Drob: Characterisation and corrosion resistance of the electrodeposited hydroxyapatite and bovine serum albumin/hydroxyapatite films on Ti–6Al–4V–1Zr alloy surface. Corros. Sci. 53, 992–999 (2011).
V.K. Truong, R. Lapovok, Y.S. Estrin, S. Rundell, J.Y. Wang, C.J. Fluke, R.J. Crawford, and E.P. Ivanova: The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 31, 3674–3683 (2010).
D.M. Medeiros, A. Plattner, D. Jennings, and B. Stoecker: Bone morphology, strength, and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J. Nutr. 132, 3135–3141 (2002).
S. Katsumata, R. Tsuboi, M. Uehara, and K. Suzuki: Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci., Biotechnol., Biochem. 70, 2547–2550 (2006).
S. Bose, D. Banerjee, S. Robertson, and S. Vahabzadeh: Enhanced in vivo bone and blood vessel formation by iron oxide and silica doped 3D printed tricalcium phosphate scaffolds. Ann. Biomed. Eng. 46, 1241–1253 (2018).
K.J. Kazmierski, G.K. Ogilvie, M.J. Fettman, S.E. Lana, J.A. Walton, R.A. Hansen, K.L. Richardson, D.W. Hamar, C.L. Bedwell, G. Andrews, and S. Chavey: Serum zinc, chromium, and iron concentrations in dogs with lymphoma and osteosarcoma. J. Vet. Intern. Med. 15, 585–588 (2001).
G-H. Yu, L. Fu, J. Chen, F. Wei, and W-X. Shi: Decreased expression of ferritin light chain in osteosarcoma and its correlation with epithelial-mesenchymal transition. Eur. Rev. Med. Pharmacol. Sci. 22, 2580–2587 (2018).
P. Li, X. Zheng, K. Shou, Y. Niu, C. Jian, Y. Zhao, W. Yi, X. Hu, and A. Yu: The iron chelator Dp44mT suppresses osteosarcoma’s proliferation, invasion, and migration: In vitro and in vivo. Am. J. Transl. Res. 82, 5370–5385 (2016).
Acknowledgments
We acknowledge Dr. Michael HajiSheikh and Mr. Gregg Westberg (Microelectronics Research and Development Lab) at Northern Illinois University and Dr. Q. Jane Wang at Northwestern University. We also thank College of Engineering and Engineering Technology and Division of Research and Innovation Partnerships for supporting this research. N.S. Lin is a high-school summer research volunteer from Milton High School, Milton, GA30004, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duvvuru, M.K., Wu, L., Lin, N.S. et al. Surface treatment of titanium by anodization and iron deposition: mechanical and biological properties. Journal of Materials Research 35, 1290–1297 (2020). https://doi.org/10.1557/jmr.2020.107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2020.107