Abstract
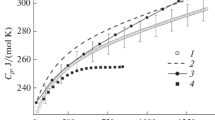
Using the data obtained by Knudsen effusion mass spectrometry, the standard formation thermodynamic properties of La2Hf2O7, Nd2Hf2O7, and Gd2Hf2O7 were calculated in the present study at high temperatures. Based on the results obtained, it was shown that the standard formation Gibbs energies of La2Hf2O7, Nd2Hf2O7, and Gd2Hf2O7 from the elements at the temperature 2445 K were consistent with the empirical rule concerning decrease of stability of pyrochlore hafnate phase with decrease in lanthanoid ionic radius. The La2Hf2O7 and Gd2Hf2O7 heat capacities were obtained in the present study by differential scanning calorimetry. These data were used along with those found earlier to evaluate the standard formation Gibbs energies of La2Hf2O7 and Gd2Hf2O7 from the elements at the temperature 298 K, which equal (−3937 ± 10) kJ/mol and (−3895 ± 10) kJ/mol, respectively. The thermodynamic properties of La2Hf2O7, Nd2Hf2O7, and Gd2Hf2O7 estimated in a wide temperature range allowed consideration of reliability of data available in the literature.



Similar content being viewed by others
References
V.L. Stolyarova: Mass spectrometric thermodynamic studies of oxide systems and materials. Russ. Chem. Rev. 85, 60 (2016).
V.B. Glushkova, M.V. Kravchinskaya, A.K. Kuznetsov, and P.A. Tikhonov: Hafnium Dioxide and its Compounds with Rare Earth Oxides (Nauka, Leningrad, 1984).
X.Q. Cao, R. Vassen, and D. Stoever: Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 24, 1 (2004).
J.R. Nicholls, K.J. Lawson, A. Johnstone, and D.S. Rickerby: Methods to reduce the thermal conductivity of EB-PVD TBCs. Surf. Coat. Technol. 151–152, 383 (2002).
D.A. Chubarov and P.V. Matveev: New ceramic materials for thermal barrier coatings using in GTE turbine blades. Aviac. Mater. Tehnol., 43 (2013).
E.N. Kablov and V.N. Toloraiya: VIAM—Founder of domestic technology for casting single-crystal turbine blades of GTE and GTS. Aviac. Mater. Tehnol., 105 (2012).
D.R. Clarke and S.R. Phillpot: Thermal barrier coating materials. Mater. Today 8, 22 (2005).
R.A. Miller: Thermal barrier coatings for aircraft engines: History and directions. J. Therm. Spray Technol. 6, 35 (1997).
A. Navrotsky: Thermochemical insights into refractory ceramic materials based on oxides with large tetravalent cations. J. Mater. Chem. 15, 1883 (2005).
C.E. Curtis, L.M. Doney, and J.R. Johnson: Some properties of hafnium oxide, hafnium silicate, calcium hafnate, and hafnium carbide. J. Am. Ceram. Soc. 37, 458 (1954).
H. Ibégazène, S. Alpérine, and C. Diot: Yttria-stabilized hafnia-zirconia thermal barrier coatings: The influence of hafnia addition on TBC structure and high-temperature behaviour. J. Mater. Sci. 30, 938 (1995).
J. Wang, H.P. Li, and R. Stevens: Hafnia and hafnia-toughened ceramics. J. Mater. Sci. 27, 5397 (1992).
E.M. Larsen: Recent advances in the chemistry of zirconium and hafnium. J. Chem. Educ. 28, 529 (1951).
A.V. Shlyakhtina and L.G. Shcherbakova: New solid electrolytes of the pyrochlore family. Russ. J. Electrochem. 48, 1 (2012).
F.A. López-Cota, N.M. Cepeda-Sánchez, J.A. Díaz-Guillén, O.J. Dura, M.A. López de la Torre, M. Maczka, M. Ptak, and A.F. Fuentes: Electrical and thermophysical properties of mechanochemically obtained lanthanide hafnates. J. Am. Ceram. Soc. 100, 1994 (2017).
J. Müller, U. Schröder, T.S. Böscke, I. Müller, U. Böttger, L. Wilde, J. Sundqvist, M. Lemberger, P. Kücher, T. Mikolajick, and L. Frey: Ferroelectricity in yttrium-doped hafnium oxide. J. Appl. Phys. 110, 114113 (2011).
S. Mueller, C. Adelmann, A. Singh, S. Van Elshocht, U. Schroeder, and T. Mikolajick: Ferroelectricity in Gd-doped HfO2 thin films. ECS J. Solid State Sci. Technol. 1, N123 (2012).
L. Chen, Y. Xu, Q.Q. Sun, P. Zhou, P.F. Wang, S.J. Ding, and D.W. Zhang: Atomic-layer-deposited HfLaO-based resistive switching memories with superior performance. IEEE Electron Device Lett. 31, 1296 (2010).
V.A. Vorozhtcov, V.L. Stolyarova, S.I. Lopatin, E.P. Simonenko, N.P. Simonenko, K.A. Sakharov, V.G. Sevastyanov, and N.T. Kuznetsov: Vaporization and thermodynamic properties of lanthanum hafnate. J. Alloys Compd. 735, 2348 (2018).
V.G. Sevastyanov, E.P. Simonenko, N.P. Simonenko, V.L. Stolyarova, S.I. Lopatin, and N.T. Kuznetsov: Synthesis, vaporization and thermodynamic properties of superfine Nd2Hf2O7 and Gd2Hf2O7. Eur. J. Inorg. Chem. 2013, 4636 (2013).
V.G. Sevastyanov, E.P. Simonenko, D.V. Sevastyanov, N.P. Simonenko, V.L. Stolyarova, S.I. Lopatin, and N.T. Kuznetsov: Synthesis, vaporization, and thermodynamics of ultrafine Nd2Hf2O7 powders. Russ. J. Inorg. Chem. 58, 1 (2013).
V.L. Stolyarova, V.A. Vorozhtcov, and S.I. Lopatin: Percularities of thermodynamic description of systems based on hafnia and rare earth oxides at high temperatures. Trans. Kola Sci. Cent. 9, 104 (2018).
R. Babu and K. Nagarajan: Calorimetric measurements on rare earth pyrohafnates RE2Hf2O7 (RE = La, Eu, Gd). J. Alloys Compd. 265, 137 (1998).
S.V. Ushakov, A. Navrotsky, J.A. Tangeman, and K.B. Helean: Energetics of defect fluorite and pyrochlore phases in lanthanum and gadolinium hafnates. J. Am. Ceram. Soc. 90, 1171 (2007).
A.R. Kopan’, M.P. Gorbachuk, S.M. Lakiza, and Y.S. Tishchenko: Calorimetric study of the La2Hf2O7 heat capacity in the range 57–302 K. Powder Metall. Met. Ceram. 54, 696 (2016).
V.L. Stolyarova, V.A. Vorozhtcov, S.I. Lopatin, and A.L. Shilov: Thermodynamic properties of the La2O3–HfO2 system at high temperatures. Thermochim. Acta 668, 87 (2018).
Y.N. Paputsky, V.A. Krzhizhanovskaya, and V.B. Glushkova: The enthalpy of formation of hafnates and zirconates of rare earth elements. Izv. Akad. Nauk SSSR, Neorg. Mater. 10, 1551 (1974).
P.A. Arseniev, V.B. Glushkova, A.A. Evdokimov, E.K. Keller, V.B. Kravchenko, M.V. Kravchinskaya, V.A. Krzhizhanovskaya, A.K. Kuznetsov, K.M. Kurbanov, A.V. Potemkin, P.A. Tikhonov, and M.N. Tseytlin: Compounds of Rare Earth Elements. Zirconates, Hafnates, Niobates, Tantalates and Antimonates (Nauka, Moscow, 1985).
E.N. Kablov, V.L. Stolyarova, S.I. Lopatin, V.A. Vorozhtcov, F.N. Karachevtsev, and Y.I. Folomeikin: High-temperature mass spectrometric study of the vaporization processes and thermodynamic properties in the Gd2O3–Y2O3–HfO2 system. Rapid Commun. Mass Spectrom. 31, 1137 (2017).
E.N. Kablov, V.L. Stolyarova, V.A. Vorozhtcov, S.I. Lopatin, O.В. Fabrichnaya, M.O. Ilatovskaya, and F.N. Karachevtsev: Vaporization and thermodynamics of ceramics based on the La2O3–Y2O3–HfO2 system studied by the high-temperature mass spectrometric method. Rapid Commun. Mass Spectrom. 32, 686 (2018).
A.N. Belov and G.A. Semenov: Mass-spectrometric investigation of evaporation of ternary solid solutions of ZrO2–HfO2–Y2O3 system. Izv. Akad. Nauk SSSR, Neorg. Mater. 25, 994 (1989).
V.G. Sevastyanov, E.P. Simonenko, N.P. Simonenko, V.L. Stolyarova, S.I. Lopatin, and N.T. Kuznetsov: Synthesis, vaporization and thermodynamics of ceramic powders based on the Y2O3–ZrO2–HfO2 system. Mater. Chem. Phys. 153, 78 (2015).
V.A. Vorozhtcov, A.L. Shilov, and V.L. Stolyarova: Features of thermodynamic description of properties of Gd2O3–Y2O3–HfO2 based ceramics. Russ. J. Gen. Chem. 89, 452 (2019).
A.L. Shilov, V.L. Stolyarova, V.A. Vorozhtcov, and S.I. Lopatin: Thermodynamic description of the Gd2O3–Y2O3–HfO2 and La2O3–Y2O3–HfO2 systems at high temperatures. Calphad 65, 165 (2019).
A.N. Belov and G.A. Semenov: Thermodynamics of binary solid solutions of zirconium, hafnium and yttrium oxides from high temperature mass spectrometry data. Russ. J. Phys. Chem. 59, 589 (1985).
K.N. Marushkin and A.S. Alikhanyan: A study of the quasibinary systems HfO2–ZrO2, ZrO2–Y2O3, and HfO2–Y2O3. Russ. J. Inorg. Chem. 36, 2637 (1991).
E.N. Kablov, Y.I. Folomeikin, V.L. Stolyarova, and S.I. Lopatin: Mass-spectrometric study of vaporization of high refractory ceramics. Dokl. Phys. Chem. 463, 150 (2015).
G.A. Semenov, L.A. Kuligina, G.A. Teterin, E.M. Menchuk, and T.M. Shkol’nikov: Mass-spectrometric investigation of components of solid solutions in HfO2–Sc2O3 system. Sov. Prog. Chem. 52, 1 (1986).
G.A. Semenov, A.N. Belov, V.N. Baydin, P.I. Ivanauskas, V.V. Vyšniauskas, J.S. Majauskas, A.G. Karaulov, and N.V. Taranuha: Sublimation of refractory ceramics based on the solid solutions in the systems HfO2–ZrO2 and Y2O3–ZrO2. Work. Acad. Sci. Lith. SSR. Ser. B 5, 115 (1977).
N.I. Ionov: Ionizatsiya molekul KJ, NAJ i CsCl elektronami (Ionization of KI, NaI, and CsCl molecules by electrons). Dokl. Akad. Nauk SSSR 59, 467 (1948).
V.L. Stolyarova and G.A. Semenov: Mass Spectrometric Study of the Vaporization of Oxide Systems (John Wiley, Chichester, 1994).
V.L. Stolyarova: Review KEMS 2012 till 2017. Calphad 64, 258 (2019).
E.N. Kablov, V.L. Stolyarova, S.I. Lopatin, V.A. Vorozhtcov, F.N. Karachevtsev, and Y.I. Folomeikin: Mass spectrometric study of thermodynamic properties in the Gd2O3–Y2O3 system at high temperatures. Rapid Commun. Mass Spectrom. 31, 538 (2017).
F. Kohler: Zur Berechnung der thermodynamischen Daten eines ternären Systems aus den zugehörigen binären Systemen. Monatsh. Chem. 91, 738 (1960).
J.A. Barker: Cooperative orientation effects in solutions. J. Chem. Phys. 20, 1526 (1952).
R. Kandan, B. Prabhakara Reddy, G. Panneerselvam, and U.K. Mudali: Enthalpy measurements on rare earth hafnates RE2O3·2HfO2 (s) (RE = Sm, Eu, Dy). J. Therm. Anal. Calorim. 131, 2687 (2018).
S. Lutique, P. Javorskỳ, R.J.M. Konings, J-C. Krupa, A.C.G. van Genderen, J.C. van Miltenburg, and F. Wastin: The low-temperature heat capacity of some lanthanide zirconates. J. Chem. Thermodyn. 36, 609 (2004).
E.R. Andrievskaya: Phase equilibria in the refractory oxide systems of zirconia, hafnia and yttria with rare-earth oxides. J. Eur. Ceram. Soc. 28, 2363 (2008).
L.V. Gurvich, I.V. Veitz, V.A. Medvedev, G.A. Bergman, V.S. Jungman, G.A. Khachkuruzov, and V.S. Iorish: Thermodynamic Properties of Individual Substances, Vol. 4 (Nauka, Moscow, 1982).
R.J.M. Konings, O. Beneš, A. Kovács, D. Manara, D. Sedmidubský, L. Gorokhov, V.S. Iorish, V. Yungman, E. Shenyavskaya, and E. Osina: The thermodynamic properties of the f-elements and their compounds. Part 2. The lanthanide and actinide oxides. J. Phys. Chem. Ref. Data 43, 013101 (2014).
R.D. Shannon: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751 (1976).
P. Simoncic and A. Navrotsky: Systematics of phase transition and mixing energetics in rare earth, yttrium, and scandium stabilized zirconia and hafnia. J. Am. Ceram. Soc. 90, 2143 (2007).
B.P. Mandal and A.K. Tyagi: Preparation and high temperature-XRD studies on a pyrochlore series with the general composition Gd2−xNdxZr2O7. J. Alloys Compd. 437, 260 (2007).
R.J.M. Konings and O. Beneš: The thermodynamic properties of the f-elements and their compounds. I. The lanthanide and actinide metals. J. Phys. Chem. Ref. Data 39, 043102 (2010).
K.A. Sakharov, E.P. Simonenko, N.P. Simonenko, M.L. Vaganova, Y.E. Lebedeva, A.S. Chaynikova, I.V. Osin, O.Y. Sorokin, D.V. Grashchenkov, V.G. Sevastyanov, N.T. Kuznetsov, and E.N. Kablov: Glycol–citrate synthesis of fine-grained oxides La2−xGdxZr2O7 and preparation of corresponding ceramics using FAST/SPS process. Ceram. Int. 44, 7647 (2018).
N.P. Simonenko, K.A. Sakharov, E.P. Simonenko, V.G. Sevastyanov, and N.T. Kuznetsov: Glycol–citrate synthesis of ultrafine lanthanum zirconate. Russ. J. Inorg. Chem. 60, 1452 (2015).
V.V. Popov, A.P. Menushenkov, A.A. Yaroslavtsev, Y.V. Zubavichus, B.R. Gaynanov, A.A. Yastrebtsev, D.S. Leshchev, and R.V. Chernikov: Fluorite–pyrochlore phase transition in nanostructured Ln2Hf2O7 (Ln = La–Lu). J. Alloys Compd. 689, 669 (2016).
Acknowledgments
This study was financially supported by the Russian Foundation for Basic Research through Grant Nos. 16-03-00940 and 19-03-00721. Determination of the La2Hf2O7 and Gd2Hf2O7 heat capacities was performed at the Thermogravimetric and Calorimetric Research Center of the Research Park of Saint Petersburg State University. Authors are very grateful to K.A. Sakharov, V.G. Sevastyanov, and N.T. Kuznetsov for supplying the La2Hf2O7 and Gd2Hf2O7 synthesized and identified samples used in the present study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
43578_2019_34193326_MOESM1_ESM.doc
Supplementary information: Thermodynamic properties of lanthanum, neodymium, gadolinium hafnates (Ln2Hf2O7) using the KEMS results (approximately 409 KB)
Rights and permissions
About this article
Cite this article
Vorozhtcov, V.A., Stolyarova, V.L., Chislov, M.V. et al. Thermodynamic properties of lanthanum, neodymium, gadolinium hafnates (Ln2Hf2O7): Calorimetric and KEMS studies. Journal of Materials Research 34, 3326–3336 (2019). https://doi.org/10.1557/jmr.2019.206
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2019.206