Abstract
Lithium–sulphur (Li–S) batteries are one of the most promising candidates for the next generation of energy storage systems to alleviate the energy crisis. However, Li–S batteries’ commercialization faces the challenges of low active materials utilization, poor cycling life, and low energy density. Recently, tremendous progress has been achieved in improving the electrode performances and tap density by using the nanostructured metal compounds in Li–S batteries. In this review, we not only present the latest various nanostructured metal compounds applications in Li–S batteries, including metal oxides, metal sulphides, metal carbides, metal nitrides, and metal organic frameworks, but also we focus on the interaction mechanisms between these polar metal compounds with polysulphides. The issues and bottlenecks of these metal compounds are concluded and the corresponding available solutions to address these issues are proposed. This systematic discussion and proposed strategies can offer avenues to the practical application of Li–S batteries in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. INTRODUCTION
Li–S batteries are regarded as one of the most promising rechargeable batteries that could meet the demands of the electric vehicles and grid energy storage due to its high theoretical specific energy and volumetric energy density of approximately 2600 W h/kg and 2800 W h/L,1–4 respectively. Additionally, sulfur as the electroactive material is not only nontoxic, cost-effective, and highly abundant on Earth, but also it possesses a safer operating voltage range (1.5–2.5 V versus Li/Li+).5,6 All the advantages make Li–S cells extraordinarily attractive toward researchers all over the world.
However, the early research on Li–S batteries progress slowly because it suffers several severe issues. First of all, the low electric and Li+-ionic conductivity of elemental sulfur and discharged end-product (Li2S and Li2S2), which will result in low utilization of active materials and poor coulombic efficiency.7,8 Secondly and most importantly, the discharged intermediates-polysulphides easily dissolve in the organic electrolyte.8 These polysulfide anions (Sx2−) can diffuse to the anode, causing tremendous loss of active materials, extremely low coulombic efficiency, and rapid capacity fading.6 Thirdly, the huge volume expansion/shrinkage of the sulfur electrode during charge/discharge process, leads to the electrode collapse and short cycle life.3,6
Therefore multiple and combined strategies, such as developing new electrolyte and binder,9,10 protecting the anode,11 modifying the separator12 and the sulfur cathode13,14 are used to solve the above problems. Among which, using the nanostructured materials to modify the sulfur cathode are most popular. Important progress was made by Nazar and coworkers,15 who showed that by fabricating cathodes where sulfur had been encapsulated into nanostructured mesoporous carbon, high reversible capacities and good rates can be obtained due to the enhanced conductivity and physical confinement of the polysulphides via the mesopores. Since then, various nanostructured conductive carbon, such as carbon fibers,16 graphene,17 carbon nanotubes (CNT),13 carbon spheres,18 porous carbon,8 etc., have been used to improve the sulfur utilization and extend the sulfur cathode cycling performances. However, the conjugate nonpolar carbon planes have limited sites to strongly anchor polar molecules (e.g., lithium polysulphides and (di)sulphides).19 To add anchoring sites and enhance anchoring ability of the carbon, tunable polar sites to chemically confine the polysulphides was introduced. Nanostructured carbon doped with heteroatoms (N,20 S,21 P,22 B23), and carbon modified with functional groups (amino-functionized,24 carboxyl-functioned,25 sulfonated,26 fluorinated,27 hydroxylated28) as well as combined with conductive polymers,29–31 have all been widely investigated for obtaining high-performance Li–S batteries. However the tap densities of these conductive carbon are commonly very low,32 which is not beneficial to the practical applications of S cathodes.
Recently, to increase the tap density of electrodes as well as to keep long cycle life-span, nanostructured polar metal compounds, such as metal oxides,33,34 metal hydroxides,13 metal sulphides,35,36 metal carbides,37 metal nitrides,38 and metal organic frameworks (MOFs),39 have been used as the host materials toward (poly)sulphides. A large amount of work nowadays reports that these metal compounds have much stronger adsorption ability to polysulphides compared to carbon, doped carbon, and conductive polymers.35,40–43 What’s more, compared to the carbon materials, the metal compounds’ exposed surfaces and morphologies are easier to control by chemical and physical methods.19 Thus a variety of nanostructured metal compounds were designed, such as hollow,34 porous,40 layered,35 laminar-structured,42 and so on, to effectively hold the polysulphides in Li–S batteries. However, compared to many reviews on the carbon and polymers materials used in Li–S batteries, the review on metal compounds for Li–S batteries are rare.
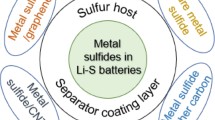
Herein, recent advances in the use of these metal compounds in Li–S batteries are reviewed. We systematically conclude metal oxides, metal hydroxides, metal sulphides, metal carbides, metal nitrides, and MOFs as sulfur host, additives, interlayers, anode/cathode/separator coatings in Li–S batteries (Scheme 1). Also we focus on how these metal compounds interact with polysulphides from both physical and chemical aspects. Meanwhile the disadvantages and bottle-neck of these metal compounds for Li–S batteries have also been proposed. Lastly we prospect feasible solving strategies and future development.
II. NANOSTRUCTURED METAL OXIDES APPLICATION IN LI–S BATTERIES
A. TixOy
TiO2, due to its natural abundance, cost-effectiveness, and polar surface, has become the most widespread and popular metal oxide applied in Li–S batteries.19 It was first used as an additive in the mesoporous carbon–sulphur cathode of Li–S batteries by Nazar’s group in 2012.44 They investigate the role of surface adsorption versus pore absorption by using three kinds of TiO2 with similar surface areas but different pore sizes (mesoporous α-TiO2 with pore size of 5.2 nm, mesoporous β-TiO2 with pore size of 9 nm, and nonporous γ-TiO2, respectively) as the additives. The electrochemical results reveal that the soluble lithium polysulphides are preferentially absorbed within the pores of the nanoporous titania while the surface adsorption also plays a more minor role. Recently, Belharouak and his co-workers also investigated the effects of nano-sized TiO2 particles as the additive on the electrochemical properties.45 The cyclic voltammetry measurements at different scan rate indicate that the addition of TiO2 helped in reducing the polarization of the sulfur electrodes. Wang and his co-workers designed a hydrogen reduction TiO2 hollow sphere (H–TiO2),46 where the TiO2 micron spheres are composed of TiO2 nano-plates. With such H–TiO2 additive, the sulfur cathode could deliver a high reversible capacity of 928.1 mA h/g after 50 charge–discharge cycles at a current density of 200 mA/g. They attributed the improvement to H–TiO2 spheres’ polar surfaces serving as the surface-bound intermediates for strong polysulphides binding.
Nanostructured TiO2 with different morphologies as the sulfur host are also widely studied.34,47–54 Cui’s group designed a sulphur–TiO2 yolk–shell nanostructure [as shown in Figs. 1(a)–1(c)],34 where the internal void space accommodates the volume expansion of sulfur and the TiO2 shell minimizes polysulfide dissolution. Accordingly, an initial specific capacity of 1030 mA h/g at 0.5 C (1 C = 1675 mA/g) and Coulombic efficiency of 98.4% over 1000 cycles were achieved. They also synthesized an inverse opal structure TiO2 to achieve both sulfur physical encapsulation and polysulphides binding.47 The inverse opal structure TiO2 after hydrogen reduction illustrated high conductivity. Furthermore, the relatively enclosed three dimensional (3D) structure [as shown in Figs. 1(d)–1(f)] provided an ideal architecture for sulfur and polysulphides confinement, which contributes to the 3D TiO2–sulphur cathode high initial capacity of 1100 mA h/g and reversible capacity of 890 mA h/g after 200 cycles at 0.5 C. Such good capacity retention for the reduced TiO2–S sample is due to (i) a significantly increased electrical conductivity achieved after hydrogen reduction; (ii) rapid electron and lithium-ion transport resulted from the 3D framework and the thin TiO2 shell; (iii) the generated oxygen vacancies promoting the interaction between the TiO2 and the sulfur, which renders the rational integration of physical confinement and chemical absorption of polysulphides in a working cell. In addition, nanotube TiO2/S composite,50 nano fibers TiO2–S composites51 and mesoporous hollow TiO2 sphere–S composites,54 porous TiO2–S composites,48,55 have also been designed and fabricated a novel cathode for Li–S batteries.
(a) Synthesis and characterization of sulphur–TiO2 yolk–shell nanostructures. (b) SEM image and (c) TEM image of as-synthesized sulphur–TiO2 yolk–shell nanostructures. Reproduced with permission from Ref. 34, Copyright 2013, Nature Publishing Group. (d) Schematic of the synthetic process that involves encapsulating sulfur nanoparticles with reduced TiO2 to form 3D sulphur–TiO2−x core–shell Nanostructures, (e) cross-sectional SEM image of the 3D ordered reduced TiO2 structure, (f) cross-sectional SEM image of the composite structure showing sulfur particles well encapsulated by the reduced TiO2 nanospheres. Reproduced with permission from Ref. 47, Copyright 2014, American Chemical Society.
Due to the insulating nature of both TiO2 and sulfur, combining conductive carbon with TiO2–S composites to further improve the electrochemical performances is becoming increasingly favorable by the researchers.54–68 Zhang’s group developed a titanium dioxide anchored on hollow carbon nanofiber hybrid nanostructure (HCNF@TiO2–S).66 The HCNF@TiO2–S composite exhibited much better electrochemical performance than the HCNF–S composite, which delivered an initial discharge capacity of 1040 mA h/g and maintained 650 mA h/g after 200 cycles at a 0.5 C rate. Tilahun and his co-workers designed hybrid nanostructured microporous carbon-mesoporous carbon doped titanium dioxide/sulfur composite (MC-meso C-doped TiO2/S).57 The incorporation of microporous carbon can effectively increase the electrical conductivity of the material by decreasing the resistance of sulfur which results in enhanced active material utilization. Simultaneously, mesoporous C-doped TiO2 nanotubes prevents the dissolution of polysulfide, and also improves the strength of the entire electrode, thereby enhancing the electrochemical performance. Yu et al., designed a TiO2–nitrogen doped graphene/sulfur (TiO2–NG/S) hybrid structures by atomic layer deposition (ALD) method.58 The nitrogen doped graphene was used as a conductive matrix and the TiO2 coating on the NG/S electrodes surface was used to inhibit lithium polysulphides shuttle. The performances of the electrodes with different cycled-TiO2 coating have been investigated. Particularly, the NG/S electrode with 20 TiO2 cycles coating illustrated a high initial specific capacity of 1070 mA h/g and a reversible capacity of 918 mA h/g even after 500 cycles at 1 C, showing excellent potential as a cathode material for Li–S batteries.
In addition to using TiO2 as the additive and host for the sulfur cathode, it is also used as an interlayer and a separator coating to enhance electrochemical performances.69–73 A TiO2 nanowire-embedded graphene (TiO2 NW/G) hybrid membrane was prepared by Manthiram’s group.74 In this hybrid membrane, the graphene with high conductivity was used as the current collector, while the TiO2 NWs were used as a polysulphides shuttling inhibitor as well as the catalyst to accelerate the polysulfide reduction and oxidation. As a result, the Li2S6 catholyte with such a hybrid membrane interlayer showed a high specific capacity of 1327 mA h/g at 0.2 C rate, a Coulombic efficiency approaching 100% and a reversible capacity of 1053 mA h/g over 200 cycles. Recently, Fanqun Li and his co-workers developed a carbonized bacterial cellulose/titania (CBC/TiO2) modified separator.75 This TiO2 modified separator could restrain the shuttle effect of Li–S cells with strong physical and chemical adsorption of polysulphides.
What’s more, Ti4O7, another titanium oxide, has also been investigated due to its positive function in Li–S batteries.76–78 Nazar’s group firstly reported that Ti4O7 has a high affinity for lithium polysulphides due to the contained polar O–Ti–O units.78 The presence of strong metal oxide-polysulfide interactions has been proved by X-ray photoelectron spectroscopy (XPS), X-ray absorption near-edge structure (XANES) and lithium polysulphides adsorption studies. Following on Cui’s group reported that, compared with the TiO2–S, the Ti4O7–S cathodes exhibited a higher reversible capacity and improved cycling performance.77 The superiorities of Ti4O7–S cathodes could be attributed to the strong adsorption of sulfur species on the low-coordinated Ti sites of Ti4O7, which was revealed by density functional theory (DFT).
As one of the most cost-effective, safe and controllable nanomaterials, TiO2 is a primary candidate for researchers’ investigation. Polysulphides are likely to be chemically bonded at oxygen defect sites and surface defect of TiO2.19 However, due to the insulation of TiO2, a conductive agent, such as CNT, graphene, etc., should be introduced rationally to generate a synergistic effect to produce high energy density and long life span Li–S batteries. Meanwhile Ti4O7, due to its low-coordinated Ti, is a promising polar host for the complex multi-electron Li–S conversion reaction.
B. MnxOy
MnO2 is usually characteristically nonstoichiometric and is deficient in oxygen atoms.19 As stated above, TiO2 with oxygen defect sites and surface defects is beneficial to trap polysulphides, thus MnO2 is also a good candidate to fabricate a high performance S cathode.14,43,52,79–81 In 2014, Nazar’s group reported δ-MnO2 nanosheets, which served as the prototype, react with initially formed lithium polysulphides to form surface-bound intermediates.81 Different from TiO2 chemical retention for the polysulphides, MnO2 chemical retention relied on mediating polysulfide redox through insoluble thiosulfate species in a two-step process, where the thiosulfate groups are first created in situ by oxidation of initially formed soluble lithium polysulfide species on the surface of ultra-thin MnO2 nanosheets, and then the surface thiosulfate groups are proposed to anchor newly formed soluble ‘higher’ polysulphides by catenating them to form polythionates and converting them to insoluble ‘lower’ polysulphides. Because of such chemical retention mechanism, the sulfur/manganese dioxide nanosheet composite with 75 wt% sulfur exhibited a reversible capacity of 1300 mA h/g at 0.2 C and a fade rate over 2000 cycles of 0.036%/cycle at 2 C. From then on, nanowire α-MnO2-coated sulfur cathode,14 core–shell sulphur-δ-MnO2 cathode,80 and core–shell γ-MnO2-coated sulfur cathode,79 have been reported in succession.
Similar to TiO2, the conductivity of MnO2 is also not good. Therefore a considerable amount of conductive additives, such as the conductive polymer or conductive carbon, should be introduced to overcome the dead active material and achieve high performances.43,82–85 Yu and her co-workers designed a S/Polypyrrole–MnO2 (S/PPy–MnO2) ternary nanostructure as shown in Fig. 2(a).43 Combining the advantages of conductive polypyrrole and MnO2, the S/Polypyrrole–MnO2 cathode with 70 wt% S content showed a reversible capacity of 550 mA h/g after 500 cycles with an extremely low decay rate of 0.07% per cycle at 1 C-rate as shown in Fig. 2(b). Lou’s group designed a hollow carbon nanofiber-MnO2 nanosheet-S (MnO2@HCF/S) nanostructure that the sulfur particle and MnO2 nanosheets were filled in the hollow carbon nanofiber as shown in Fig. 2(c). With such a unique structure, the MnO2@HCF hybrid host not only facilitated electron and ion transfer during the redox reactions, but also efficiently prevented polysulfide dissolution. As a result, the MnO2@HCF/S electrode, with 71 wt% S content in the composite and an area of sulfur mass loading of 3.5 mg/cm2, maintained a reversible capacity of 662 mA h/g at 0.5 C over 300 cycles as shown in Fig. 2(d).
(a) Illustration of the synthesis of S/PPy-MnO2 ternary composites. (b) Cycling performance of PPy-MnO2 nanotubes encapsulated sulfur electrode compared with pure PPy nanotubes encapsulated sulfur electrode at 1 C. Reproduced with permission from Ref. 43, Copyright 2016, American Chemical Society. (c) Synthesis of the MnO2@HCF/S composite. (d) Prolonged cycling performance of MnO2@HCF/S at 0.5 C and the corresponding Coulombic efficiency. Reproduced with permission from Ref. 84, Copyright 2015, Wiley-VCH.
Recently, MnO2 and graphene composites used as the interlayer for Li–S batteries have been reported.86,87 For example, Zhang et al., synthesized a free-standing MnO2 nanowires and graphene nanoscroll (GNSM) interlayer, where the weight ratio of graphene nanoscroll to MnO2 nanowires is 4:1. The insertion of the hybrid GNSM interlayer between sulfur cathode and separator could not only reduce the overall electrode resistance but also offer strong physical/chemical interactions for efficiently mitigating the shuttling of polysulphides, ensuring continuous reactivation and reutilization of the trapped sulfur active materials.
In addition, manganese monoxide (MnO) modified CNTs as sulfur host for improving the performance of Li–S batteries has been reported latterly.88 The CNTs/MnO–S cathode showed a better cycling stability over 100 cycles than CNTs–S cathodes with a same carbon/sulfur weight ratio at around 1:8, which demonstrated MnO is a potential additive for sulfur cathode to address the insufficiencies of carbon hosts.
Similar to TiO2, MnO2 also shows strong chemical adsorption for the polysulphides. However, the mechanism of the MnO2 in the sulfur cathodes was not clearly elucidated, especially for the different polymorphs of MnO2, e.g., α-MnO2, δ-MnO2, γ-MnO2. In other words, the interactions between the polysulphides and MnO2 with different crystal phases should be further explored by theoretical and experimental investigations. Meanwhile other manganese based oxide, such as MnO, Mn2O3 could be a promising sulfur host candidate. However, the working mechanisms also need to be further understood.
C. VxOy
V2O5, has been attracting much attention, as it offers the essential advantages of low cost, abundant sources, and better safety relative to commercial cathodes such as LiCoO2 and LiNiO2.89 However, recently it has received increasing attention due to its strong interaction with polysulphides.90,91 As early as 2009 and 2010, there were two works that reported V2O5–S composites as cathode materials for Li–S batteries.92,93 V2O5–S composites illustrated better electrochemical performances compared to the pure sulfur cathode, wherein the researchers attributed such improvements to the addition of V2O5 which could decrease the resistance of the composite electrode.93 Following on, Oh et al.,94 found the V2O5/carbon nano-composite as an additive to the Li2S6 polysulfide solution was highly effective at capturing these long-chain polysulphides on its surface. As a result, Liu and his co-workers directly used micrometer-scale V2O5 layer as the interlayer for Li–S batteries.91 A 5 mA h pouch cell with V2O5 interlayer could cycle 300 times over 1 year without noticeable degradation. Additionally a V2O5-decorated carbon nanofiber interlayer for suppressing self-discharge and shuttle effect has also been reported recently.90
What’s more, Nazar’s group found that by coating a layer of VOx onto CMK-3-S composites, the electrochemical reduction of polysulphides on the cathode surface is inhibited.95 Manthiram’s group tried to incorporate VO2(B) into sulfur cathodes to improve the performances but finally found VO2(B) was incompatible with the glyme-based electrolytes that are usually used in Li–S cells.96
D. SnOx
SnO2, was found to be a better conductive material than most other oxides in porous shell morphologies,97 therefore SnO2 shells with micromesopores were synthesized to load the sulfur inside by Zhang.97 The core–shell S/SnO2 composites with 66 wt% S content exhibited a high initial capacity of 1176 mA h/g at 0.5 C and retained a capacity of 736.6 mA h/g after 50 cycles. To further enhance the conductivity and thus enhance the performance, double-shell SnO2@C hollow nanospheres were designed to encapsulate sulfur by Cao.98 The S/SnO2@C composite illustrated a high reversible capacity of 616 mA h/g at 3200 mA/g after 100 cycles.
Recently, Lee’s group firstly reported multi-walled carbon nanotubes (MWCNT) filled with ordered tin-monoxide (SnO) nanoparticles as the sulfur host for high-rate lithium–sulphur batteries.99 The resulting MWCNT–SnO/S composite cathodes even exhibited a high capacity of 257.7 mA h/g at an extremely high current rate of 20 C. Such an excellent rate capability could be attributed to the dipole–dipole interaction between the SnO and polysulphides as well as the MWCNT–SnO host accommodating the volume expansion, protecting the sulfur from dissolution, and enhancing the electrical conductivity.99
E. Al2O3
Al2O3 is an electrochemically inactive and ceramic material with a low cost and natural abundance.11 It was firstly applied as the additive in the sulfur cathode to improve the electrochemical performances of Li–S batteries.100,101 Later Al2O3 was applied as the coating layer on both sulfur cathodes102–104 and separators,12,73,105 which has proved to be effective in enhancing the cycle stability of Li–S batteries. For instance, Shi’s group coated an ultrathin Al2O3 film via ALD on the graphene–sulphur (G–S) composite.104 The ALD-Al2O3 coated-G–S composite cathode delivered a high specific capacity of 646 mA h/g after 100 cycles at 0.5 C, which was about twice that of the bare G–S composite. Subsequently, Song et al., applied the Al2O3 and graphene to modify the polypropylene (GPA) separator for application in Li–S batteries as shown in Fig. 3.106 The Al2O3 coating could further enhance the thermal stability and safety of the graphene coated polypropylene (GCP) separator.
Schematic illustration of (a) the structure of GPA separator, and (b) Li–S battery with GPA separator. Reproduced with permission from Ref. 106, Copyright 2016, Elsevier.
Recently, porous Al2O3 was applied to protect the lithium anode by using a spin-coating method.11 The Al2O3 protective layer can restrict the side reactions between soluble lithium polysulphides and the lithium anode through a combination of physical separation and chemical adsorption by the Al2O3 layer, which can alleviate lithium corrosion due to polysulfide attack.
The ALD method is commonly used to deposit a very thin Al2O3 layer on the sulfur cathode or separator. The Al2O3 layer could not only enhance thermal stability of Li–S batteries, but also can decrease the risk of short circuits.106 However, the mechanism of Al2O3-polysulfide chemical bonding still needs to be studied.
F. ZnO
Zinc oxide (ZnO) is a nontoxic n-type semiconductor with a wide band gap of 3.37 eV.107 Due to the large exciton binding energy of 60 meV and the high mechanical, and thermal stabilities, ZnO is attractive for high-efficiency short-wavelength optoelectronic nanodevices.107 However, recently, ZnO attracts much attention as adsorbent for migrating polysulphides due to strong chemical bonding.13,108–110 Our group used ball-milling method to coat ZnO on the CNT–S cathode to improve the performances.13 The results showed that ZnO coated-CNT–S cathode illustrated much better cycling stability than that of CNT–S composites. While Gaoquan Shi’s group used ALD method to coat ZnO on reduced graphene oxide (RGO)–sulphur composite.108 With the ZnO coating, the RGO–S composites exhibited high discharge capacity, excellent cycling stability and good rate-performance.
Recently, inspired by the brush-like membrane of cells for nutrient adsorption, a similar structure of interlayer consisting of ZnO nanowires and conductive frameworks as shown in Fig. 4 has been designed for chemical adsorption of polysulphides by Kumar and his co-workers.108 The S/MWCNTs composite cathode with a ZnO/C interlayer exhibited a reversible capacity of 776 mA h/g after 200 cycles at 1 C with only 0.05% average capacity loss per cycle.
Schematic of the structural and chemical function of the hybrid ZnO nanowires/carbon nanofibers interlayer in Li–S batteries. Reproduced with permission from Ref. 108, Copyright 2016, Wiley-VCH.
G. Other metal oxide
Intrinsically polar metal oxides, which can interact with polar lithium polysulphides, have been widely used to modify the sulphur-based cathodes, such as representative Mg0.8Cu0.2O,92 Mg0.6Ni0.4O,111–113 Li4Ti5O12,114 BaTiO3,115 LiFePO4,116 NiFe2O4,117 Co3O4,118,119 ZrO2,120,121 MoO2,122 MoO3,123 Nb2O5,124 W18O49,125 MgO,33,126 CeO2,33,127 Fe2O3,128 La2O3,33,129 CaO,33 In2O3,130 etc. Most of them are coupled with conductive polymers or carbon materials to overcome the inferior conductivity of both themselves and sulfur. For example, Zhou et al.,121 incorporated a fine amount of ZrO2 to the holey CNT–sulphur composites. The holey CNT contributed to the good conductivity of the h-CNT/S/ZrO2 cathode, while appropriate ZrO2 loading preserved the permselective channels for Li+ intercalation/deintercalation and trapped the soluble polysulphides.
These various metal oxides used in Li–S batteries provide a new strategy for anchoring polysulphides. However, which group of metal oxides as well as the appropriate size and morphologies of the metal oxides that are most beneficial to trap the polysulfides should be further investigated. Further research is also needed to find whether the capture mechanism by various metal oxides is achieved via either monolayered or multilayered chemisorption.
III. NANOSTRUCTURED METAL HYDROXIDES APPLICATION IN LI–S BATTERIES
Metal hydroxides, filled with copious functional polar/hydrophilic groups (e.g., hydrophilic groups, surface hydroxyl groups, and so on),131 are another promising class of encapsulation materials to build better Li–S cells.
More recently, thin layered metal hydroxides, including Co(OH)2,132 Co(OH)2/layered double hydroxides,133 Ni(OH)2,13,134 and Ni3(NO3)2(OH)4131 have been used as effective encapsulation materials for sulfur cathodes. In one case, Lou’s group designed double-shelled nanocages with two shells of cobalt hydroxide and layered double hydroxides (CH@LDH) as a conceptually new sulfur host, as shown in Fig. 5(a).133 Such a hollow CH@LDH polyhedra with complex shell structures not only maximize the advantages of hollow nanostructures for encapsulating a high content of sulfur (75 wt%), but also provide sufficient self-functionalized surfaces for chemical bonding with polysulphides to suppress their outward dissolution. As a result, the resulted CH@LDH/S electrode with relatively high sulfur loading of 3 mg/cm2 showed excellent cycling stability at both 0.1 and 0.5 C over 100 cycles, much better than the reference C/S cathode as shown in Fig. 5(b).
(a) Schematic illustration of the synthesis of the CH@LDH/S composite. (b) Cycle performance comparison between CH@LDH/S and C/S. Reproduced with permission from Ref. 133, Copyright 2016, Wiley-VCH.
The transition-metal hydroxides as coatings for the sulphur-based cathodes could effectively trap the polysulphides, but the mechanisms in which the transition-metal hydroxides bind with polysulphides should be further investigated to open a new avenue for future development of high performance Li–S batteries.
IV. NANOSTRUCTURED METAL SULPHIDES APPLICATION IN LI–S BATTERIES
A. TiS2
Titanium disulfide (TiS2) is known as a cathode material in the first generation rechargeable lithium batteries.135 Due to its working voltage between 1.7 and 2.5 V, very similar to the voltage range of Li–S cells, it has become a second active component in sulfur cathode.135 By adding the layered TiS2 into the carbon-S cathodes, the power capability of Li–S cells has obviously improved.96,135
Recently, Archer’s group developed a foam TiS2 and used it to encapsulate elemental sulfur.40 This 3D hybrid cathode demonstrated high areal specific capacity (9 mA h/cm2) and high retention even at a relatively large areal mass loading of approximately 40 mg sulfur per cm2 and high current density (10 mA/cm2). Additionally, Cui’s group used a two dimensional (2D) layered TiS2 to encapsulate Li2S.35 As a result, the core–shell Li2S@TiS2 nanostructure was obtained. It also showed a very high area capacity of 3.0 mA h/cm2 under high mass Li2S loading (5.3 mg Li2S per cm2) and high-C rate conditions (4 C). That is because the TiS2 possesses a combination of high conductivity and polar Ti–S groups that can potentially interact strongly with Li2S/Li2Sn species.35,40 DFT analysis and ab initio simulations demonstrated the binding energy between Li2S and TiS2 was 10 times higher than that between Li2S and carbon-based graphene.35,40
B. CoxSy
Cobalt sulphides commonly show unique metallic or half-metallic characteristics, which means they exhibit particularly high room temperature conductivity.41,42,136 Therefore the cobalt sulphides could afford efficient electron pathways and high electrocatalytic activity for polysulfide redox reactions in aqueous solutions.41 Simultaneously cobalt sulphides can significantly enhance the redox reactivity of lithium polysulphides due to their strong chemical affinity.41,42
For example, Zhang’s group selected a cost-effective, nonporous mineral and bulk CoS2 as the additive to the carbon/sulfur composite cathodes.41 The adsorption experiment as shown in Fig. 6(a) and the First-principle calculations based on DFT as shown in Fig. 6(b) both proved the CoS2 showed far stronger affinities to the Li2S4 compared to graphene. Meanwhile Nazar’s group reported a metallic Co9S8 with an interconnected graphene-like nano-architecture as the polysulphides host.42 The first-principles calculations coupled with spectroscopic evidence also demonstrated the synergistic strong dual-interactions of polysulphides with Co9S8.
(a) Visualized adsorption of Li2S4 on graphene and pristine CoS2 with the same surface area. (b) Binding geometries and energies of a Li2S4 molecule on graphene (left, modeled as coronene) and (111) plane of CoS2 with cobalt-terminated surface (right), which is derived from theoretical calculation based on DFT. Reproduced with permission from Ref. 41, Copyright 2016, American Chemical Society.
C. MoS2
MoS2, has a typical layered structure and the spacing between the neighboring layers for bulk MoS2 is about 0.615 nm, which is significantly larger than that of graphite (0.335 nm).137 It is a good candidate to encapsulate the sulfur. Furthermore, the MoS2 showed strong interaction with polysulphides,138–141 which makes it more attractive for use in Li–S batteries.
Cui’s group made a significant breakthrough on how MoS2 binds with Li2S. They used ab initio simulations to choose the terrace and edge sites of MoS2 for binding Li2S.140 The calculation results showed that the binding energy between Li2S with MoS2 terrace site was ∼0.87 eV, slightly higher than the case of graphene (0.29 eV). While the edge sites, including Mo-edge and S-edge, binding energies were 4.48 and 2.70 eV, respectively, suggesting a large selectivity of edge versus terrace sites. In addition the Li2S was superior to bind with Mo-edge sites. Such a new discovery becomes important in metal sulphides and metal oxides materials synthesis for Li–S batteries.
D. Other metal sulphides
Apart from the metal sulphides illustrated above, various other metal sulphides, such as SnS2,142,143 WS2,144 MnS,145 FeS2,146 FeS,36 Ni2S3,36 NiS2,147 VS2,35,36 CuS,148 Cu3BiS3,149 ZrS2,35 and so on, have also been investigated as polar hosts to reveal the key parameters correlated to the energy barriers and polysulfide adsorption capability in Li–S batteries. Even though most of the metal sulphides have better conductivity compared to the metal oxides, it still can’t meet the demand for addressing internal resistance in the electrode, which is highly related to effective use of active materials.19 Thus various nanocarbon materials were combined with the metal sulphides to further improve the active materials utilization. For instance, WS2 nanosheets grown on the carbon nanofiber was used as the sulfur host.144 By combining the advantages of carbon nanofiber (excellent electronic transport of the 3D structure) and WS2 (polar adsorption of polysulphides), the resulted C@WS2/S composite still maintained with a high specific capacity of 502 mA h/g at 2 C, about 90% of its initial specific capacity.
Although adding metal sulphides into S-carbon based cathodes has remarkably enhanced the electrochemical performance, there still exists a gap between practical applications. Additionally most of the adsorption mechanism for polysulphides on metal sulphides are still not clearly and need to be deeply investigated.
V. NANOSTRUCTURED METAL CARBIDE APPLICATION IN LI–S BATTERIES
Metal carbide are an exciting family of transition-metal carbides,37,150,151 which are inherently highly conductive and possess a highly active 2D surfaces to chemically bond to intermediate polysulphides by metal–sulphur interactions.37
Nazar’s group first reported a new class of sulfur host materials—conductive MXene Ti2C.37 The MXene Ti2C, not only displays the characteristic high 2D electron conductivity of transition-metal carbides (much higher than GO), but also its exposed terminal metal sites can bind to the sulphides as revealed by X-ray photoelectron spectroscopy (XPS) analysis, with the corresponding mechanism scheme shown in Fig. 7.37 Due to these superiorities, Ti2C was highly effective as a sulfur host material for Li–S batteries, providing very stable cycling performance and high capacity even with 70 wt% S.
Replacement of the Ti–OH bond on the MXene surface with a S–Ti–C bond on heat treatment or by contact with polysulfides. Reproduced with permission from Ref. 37, Copyright 2015, Wiley-VCH.
From then on, the MXene Ti3C2,152 Fe3C,150 and TiC153 as the sulfur host have been subsequently reported. All these transition-metal carbides demonstrated the strong interatomic attraction for the polysulphides.
VI. NANOSTRUCTURED METAL NITRIDE APPLICATION IN LI–S BATTERIES
Metal nitrides couple the advantages of high electrical conductivity (batter than metal oxide and carbon) and the excellent chemical stability owing to the formation of an oxide passivation layer,38 which enable them to become potential host materials of sulfur.
Goodenough’s group reported the use of mesoporous TiN to encapsulate sulfur by a melt-diffusion.38 The resulting mesoporous TiN–S cathode delivered much better cycling stability and rate capability than both mesoporous TiO2–S and Vulcan C–S cathode. The excellent overall electrochemical performance of a TiN–S cathode can be attributed to the good conductivity, robust framework of TiN, and the strong interactions between TiN and polysulphides.38 Not long ago, Li and his co-workers reported a 3D porous vanadium nitride/graphene (VN/G) composite as a chemical bridle for the polysulphides.154 The anchoring effect of vanadium nitride was confirmed by both experimental and theoretical results as shown in Fig. 8. It can be clearly observed that the absorption peak of Li2S6 in the visible light remained in the solution with the addition of RGO but disappeared when adding the VN/G [Fig. 8(a)], indicating the strong adsorption of Li2S6 molecules to polar VN. The First-principles calculations based on DFT demonstrated the binding energy between Li2S6 and VN was 3.75 eV,154 much higher than that on pyridinic N-doped graphene. And the strong polar–polar interaction between Li2S6 and VN resulted in an obvious deformation of the Li2S6 molecule [Fig. 8(c)] compared to that of pyridinic N-doped graphene [Fig. 8(b)].
Demonstration of the strong interaction of VN/G composite with polysulphides. (a) Ultraviolet/visible absorption spectra of a Li2S6 solution before and after the addition of RGO and VN/G. Inset image shows a photograph of a Li2S6 solution before and an 2 h after the addition of graphene and VN/G. (b) Side view of a Li2S6 molecule on a nitrogen-doped graphene surface, the binding energy between Li2S6 and pyridinic N-doped graphene is calculated to be 1.07 eV. (c) Side view of a Li2S6 molecule on VN(200) surface, the binding energy between Li2S6 and VN is calculated to be 3.75 eV. Reproduced with permission from Ref. 154, Copyright 2017, Nature Publishing Group.
The pioneered works on TiN, VN, and Mo2N have shown the improved performances of Li–S batteries by their high conductivity and strong chemical anchor functions,38,154,155 which opens a new direction of metal nitrides for energy storage.
VII. NANOSTRUCTURED MOFs APPLICATION IN LI–S BATTERIES
MOFs are a class of porous materials assembled by connecting metal ions and organic linkers with tremendous extensiveness in variety and multiplicity.156 They usually have even richer pore structure and larger specific surface area than porous carbon.157 Thus, Tarascon pioneered the use of the MOF named MIL-100(Cr) as host material for sulfur impregnation.158 However, the confinement of polysulphides was mainly attributed to the bimodal pores of MIL100(Cr) rather than weak binding by the oxygenated framework even though the cycling performances of sulfur cathode were improved. Since then, various groups have demonstrated that other MOFs can be adopted.39,156,157,159–161
Among which, Xiao’s group proposed a mechanism of Lewis acid–base interactions between polysulphides and MOF.39 They used the Ni-MOF with interwoven mesopores (∼2.8 nm) and micropores (∼1.4 nm) as an ideal matrix to confine polysulphides. The Ni-MOF is constructed with coordinated Ni(II), which is a moderate/soft Lewis acid. The Lewis acidic Ni(II) center is inclined to coordinate with soluble polysulphides (Sx2−) anion (soft Lewis base) as axial ligand, which could effectively capture the soluble polysulphides within the cathode. To better understand the mechanism, the First-principles calculations based on DFT were performed by their group. The calculation results showed that only the S atom on one end point of polysulfide chain bound to the Ni-MOF with the corresponding interaction mechanism scheme shown in Fig. 9(a). Further binding energy calculation demonstrated the binding energies between Ni-MOF and polysulphides were higher than those between Co-MOF and polysulphides as shown in Fig. 9(b), which is consistent with the experiment results.
(a) Schematic diagram illustrating the interaction between polysulfides (e.g., Li2S8/Li2S6/Li2S4, and so forth) and paddle-wheel unit in NiMOF. C, O, N, S, Li, and Ni atoms are represented by gray, red, blue, yellow, pink, and green spheres, respectively. (b) Comparison of binding energies of lithium polysulfides to Ni-MOF or Co-MOF. Reproduced with permission from Ref. 39, Copyright 2016, American Chemical Society.
The fundamental understanding from Lewis acid–base interactions may provide new insights and opportunities to develop MOFs application in Li–S battery technology.
VIII. SUMMARY AND PERSPECTIVE
In summary, this comprehensive review has systematically demonstrated nearly all of the recent metal compounds from single-functional conductive additives to multifunctional key components in the Li–S battery. Although it is hard to foresee what the best metal compounds for Li–S batteries exactly will be, some key features can still be predicted based on current research and knowledge. First of all, the metal compounds should have strong interactions with polysulphides. According to the relative research by Nazar,162 the metal oxides with a moderate voltage (versus Li/Li+), which form surface-bound thiosulfate via redox, are the most suitable for chemically binding polysulphides. TiO2, and MnO2 both display these characteristics. Secondly, when polysulphides are anchored on a conductive substrate, they receive electrons more easily which accelerates the kinetics of the polysulphide redox reactions. Thus, metal compounds with good conductivity, such as CoS2 (6700 S/cm),41 TiC (104 S/cm),153 are better candidates for Li–S batteries. However, most metal compounds should still be coupled with conductive polymers or carbon materials to enhance the overall conductivity of the cathode rather than relying on the intrinsic conductivity to attain the best service performances in the Li–S batteries. Thirdly polar metal compounds, which can also serve as the electrocatalyst to facilitate the polysulphides redox reactions and increase the active materials utilization is preferable, of which VN,154 CoS2,41 and MnO281 have been reported. Finally, the nanostructures of metal compounds, i.e., surface area, pore size, pore volume, as well as particle size are also important factors which influence the performance of Li–S batteries.19 Generally the metal compounds with smaller size,69 high surface area and pore volume,98 and abundant micro/mesopores49,57 are beneficial to the cathode.
Even though tremendous progress in improving the electrochemical performance and understanding the reaction mechanisms of Li–S batteries have been achieved, Li–S batteries are far from being ready for practical use at this stage. Most of the reports on metal compounds-sulphur-based cathodes displayed an areal sulfur loading of 0.3–1.5 mg/cm2,19 which is far less than the requirement of 5.0 mg/cm2 for practical cells with high energy density of more than 300 W h/kg.19,163,164 Although some of the carbon–S cathode with an areal sulfur loading has exceeded 5 mg/cm2,165–167 the cycling stability is not ideal. Therefore discovering how to balance the high tap density of metal compounds with the good conductivity of carbon in the nanostructured sulfur cathode is required to realise the practical applications. The 3D hybrid electrode with heteroatoms doped/functioned carbon and porous/hollow polar metal compounds is promising to fabricate Li–S cells with high energy density and long cycle life. It is believed that Li–S batteries could be a promising and practical technology for the applications in transportation and large-scale grid energy storage in the near future.
References
P.G. Bruce, S.A. Freunberger, L.J. Hardwick, and J.M. Tarascon: Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11 (1), 19 (2012).
X.X. Gu, S.Q. Zhang, and Y.L. Hou: Graphene-based sulfur composites for energy storage and conversion in Li–S batteries. Chin. J. Chem. 34 (1), 11 (2016).
M.K. Song, E.J. Cairns, and Y. Zhang: Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities. Nanoscale 5 (6), 2186 (2013).
Y. Yang, G. Zheng, and Y. Cui: Nanostructured sulfur cathodes. Chem. Soc. Rev. 42 (7), 3018 (2013).
L. Chen and L.L. Shaw: Recent advances in lithium–sulfur batteries. J. Power Sources 267, 770 (2014).
X. Gu, L. Hencz, and S. Zhang: Recent development of carbonaceous materials for lithium–sulphur batteries. Batteries 2 (4), 33 (2016).
J. Liang, Z-H. Sun, F. Li, and H-M. Cheng: Carbon materials for Li–S batteries: Functional evolution and performance improvement. Energy Storage 2, 76 (2016).
X. Gu, Y. Wang, C. Lai, J. Qiu, S. Li, Y. Hou, W. Martens, N. Mahmood, and S. Zhang: Microporous bamboo biochar for lithium–sulfur batteries. Nano Res. 8 (1), 129 (2015).
J. Liu, D.G.D. Galpaya, L. Yan, M. Sun, Z. Lin, C. Yan, C. Liang, and S. Zhang: Exploiting a robust biopolymer network binder for an ultrahigh-areal-capacity Li–S battery. Energy Environ. Sci. 10, 750–755 (2017).
L. Suo, Y.S. Hu, H. Li, M. Armand, and L. Chen: A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
H-K. Jing, L-L. Kong, S. Liu, G-R. Li, and X-P. Gao: Protected lithium anode with porous Al2O3 layer for lithium–sulfur battery. J. Mater. Chem. A 3 (23), 12213 (2015).
G.C. Li, H.K. Jing, Z. Su, C. Lai, L. Chen, C.C. Yuan, H.H. Li, and L.L. Xiang: A hydrophilic separator for high performance lithium sulfur batteries. J. Mater. Chem. A 3, 11014 (2015).
X. Gu, C-J. Tong, B. Wen, L-M. Liu, C. Lai, and S. Zhang: Ball-milling synthesis of ZnO@sulphur/carbon nanotubes and Ni(OH)2@sulphur/carbon nanotubes composites for high-performance lithium–sulphur batteries. Electrochim. Acta 196, 369 (2016).
J. Lee, T. Hwang, Y. Lee, J.K. Lee, and W. Choi: Coating of sulfur particles with manganese oxide nanowires as a cathode material in lithium–sulfur batteries. Mater. Lett. 158, 132 (2015).
X. Ji, K.T. Lee, and L.F. Nazar: A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 8 (6), 500 (2009).
X. Gu, C. Lai, F. Liu, W. Yang, Y. Hou, and S. Zhang: A conductive interwoven bamboo carbon fiber membrane for Li–S batteries. J. Mater. Chem. A 3 (18), 9502 (2015).
M-Q. Zhao, Q. Zhang, J-Q. Huang, G-L. Tian, J-Q. Nie, H-J. Peng, and F. Wei: Unstacked double-layer templated graphene for high-rate lithium–sulphur batteries. Nat. Commun. 5, 3410 (2014).
C. Zhang, H.B. Wu, C. Yuan, Z. Guo, and X.D. Lou: Confining sulfur in double-shelled hollow carbon spheres for lithium–sulfur batteries. Angew. Chem., Int. Ed. 124, 9730 (2012).
X. Liu, J.Q. Huang, Q. Zhang, and L. Mai: Nanostructured metal oxides and sulfides for lithium–sulfur batteries. Adv. Mater. 29, 1601759–1601784 (2017).
X. Gu, C.J. Tong, S. Rehman, L.M. Liu, Y. Hou, and S. Zhang: Multifunctional nitrogen-doped loofah sponge carbon blocking layer for high-performance rechargeable lithium batteries. ACS Appl. Mater. Interfaces 8 (25), 15991 (2016).
L-B. Xing, K. Xi, Q. Li, Z. Su, C. Lai, X. Zhao, and R.V. Kumar: Nitrogen, sulfur-codoped graphene sponge as electroactive carbon interlayer for high-energy and -power lithium–sulfur batteries. J. Power Sources 303, 22 (2016).
X. Gu, C-J. Tong, C. Lai, J. Qiu, X. Huang, W. Yang, B. Wen, L-M. Liu, Y. Hou, and S. Zhang: Porous nitrogen and phosphorous dual doped graphene blocking layer for high performance Li–S batteries. J. Mater. Chem. A 3, 16670 (2015).
C.P. Yang, Y.X. Yin, H. Ye, K.C. Jiang, J. Zhang, and Y.G. Guo: Insight into the effect of boron doping on sulfur/carbon cathode in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 6 (11), 8789 (2014).
Z. Wang, Y. Dong, H. Li, Z. Zhao, H.B. Wu, C. Hao, S. Liu, J. Qiu, and X.W. Lou: Enhancing lithium–sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 5, 5002 (2014).
L. Wang, Z. Dong, D. Wang, F. Zhang, and J. Jin: Covalent bond glued sulfur nanosheet-based cathode integration for long-cycle-life Li–S batteries. Nano Lett. 13 (12), 6244 (2013).
L. Zhou, X. Lin, T. Huang, and A. Yu: Binder-free phenyl sulfonated graphene/sulfur electrodes with excellent cyclability for lithium sulfur batteries. J. Mater. Chem. A 2 (14), 5117 (2014).
A. Vizintin, M.U.M. Patel, B. Genorio, and R. Dominko: Effective separation of lithium anode and sulfur cathode in lithium–sulfur batteries. ChemElectroChem 1 (6), 1040 (2014).
C. Zu and A. Manthiram: Hydroxylated graphene–sulfur nanocomposites for high-rate lithium–sulfur batteries. Adv. Energy Mater. 3, 1008 (2013).
L. Yin, J. Wang, F. Lin, J. Yang, and Y. Nuli: Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li–S batteries. Energy Environ. Sci. 5 (5), 6966 (2012).
W. Zhou, Y. Yu, H. Chen, F.J. DiSalvo, and H.D. Abruna: Yolk–shell structure of polyaniline-coated sulfur for lithium–sulfur batteries. J. Am. Chem. Soc. 135 (44), 16736 (2013).
W. Li, Q. Zhang, G. Zheng, Z.W. Seh, H. Yao, and Y. Cui: Understanding the role of different conductive polymers in improving the nanostructured sulfur cathode performance. Nano Lett. 13 (11), 5534 (2013).
G. Babu and L.M. Reddy Arava: Graphene-decorated graphite–sulfur composite as a high-tap-density electrode for Li–S batteries. RSC Adv. 5 (59), 47621 (2015).
X. Tao, J. Wang, C. Liu, H. Wang, H. Yao, G. Zheng, Z.W. Seh, Q. Cai, W. Li, G. Zhou, C. Zu, and Y. Cui: Balancing surface adsorption and diffusion of lithium–polysulfides on nonconductive oxides for lithium–sulfur battery design. Nat. Commun. 7, 11203 (2016).
Z.W. Seh, W. Li, J.J. Cha, G. Zheng, Y. Yang, M.T. McDowell, P.C. Hsu, and Y. Cui: Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 4, 1331 (2013).
Z.W. Seh, J.H. Yu, W. Li, P.C. Hsu, H. Wang, Y. Sun, H. Yao, Q. Zhang, and Y. Cui: Two-dimensional layered transition metal disulphides for effective encapsulation of high-capacity lithium sulphide cathodes. Nat. Commun. 5, 5017 (2014).
G. Zhou, H. Tian, Y. Jin, X. Tao, B. Liu, R. Zhang, Z.W. Seh, D. Zhuo, Y. Liu, J. Sun, J. Zhao, C. Zu, D.S. Wu, Q. Zhang, and Y. Cui: Catalytic oxidation of Li2S on the surface of metal sulfides for Li–S batteries. Proc. Natl. Acad. Sci. U. S. A. 114, 840–845 (2017).
X. Liang, A. Garsuch, and L.F. Nazar: Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium–sulfur batteries. Angew. Chem., Int. Ed. 54 (13), 3907 (2015).
Z. Cui, C. Zu, W. Zhou, A. Manthiram, and J.B. Goodenough: Mesoporous titanium nitride-enabled highly stable lithium–sulfur batteries. Adv. Mater. 28 (32), 6926 (2016).
J. Zheng, J. Tian, D. Wu, M. Gu, W. Xu, C. Wang, F. Gao, M.H. Engelhard, J.G. Zhang, J. Liu, and J. Xiao: Lewis acid–base interactions between polysulfides and metal organic framework in lithium sulfur batteries. Nano Lett. 14 (5), 2345 (2014).
L. Ma, S. Wei, H.L. Zhuang, K.E. Hendrickson, R.G. Hennig, and L.A. Archer: Hybrid cathode architectures for lithium batteries based on TiS2 and sulfur. J. Mater. Chem. A 3 (39), 19857 (2015).
Z. Yuan, H.J. Peng, T.Z. Hou, J.Q. Huang, C.M. Chen, D.W. Wang, X.B. Cheng, F. Wei, and Q. Zhang: Powering lithium–sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts. Nano Lett. 16 (1), 519 (2016).
Q. Pang, D. Kundu, and L.F. Nazar: A graphene-like metallic cathode host for long-life and high-loading lithium–sulfur batteries. Mater. Horiz. 3 (2), 130 (2016).
J. Zhang, Y. Shi, Y. Ding, W. Zhang, and G. Yu: In situ reactive synthesis of polypyrrole–MnO2 coaxial nanotubes as sulfur hosts for high-performance lithium–sulfur battery. Nano Lett. 16 (11), 7276 (2016).
S. Evers, T. Yim, and L.F. Nazar: Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li–S battery. J. Phys. Chem. C 116 (37), 19653 (2012).
R. Xu, J.C.M. Li, J. Lu, K. Amine, and I. Belharouak: Demonstration of highly efficient lithium–sulfur batteries. J. Mater. Chem. A 3 (8), 4170 (2015).
Z.Z. Yang, H.Y. Wang, L. Lu, C. Wang, X.B. Zhong, J.G. Wang, and Q.C. Jiang: Hierarchical TiO2 spheres as highly efficient polysulfide host for lithium–sulfur batteries. Sci. Rep. 6, 22990 (2016).
Z. Liang, G. Zheng, W. Li, Z.W. Seh, H. Yao, K. Yan, D. Kong, and Y. Cui: Sulfur cathodes with hydrogen reduced titanium dioxide inverse opal structure. ACS Nano 8 (5), 5249 (2014).
J. Li, J. Guo, J. Deng, and Y. Huang: Enhanced electrochemical performance of lithium–sulfur batteries by using mesoporous TiO2 spheres as host materials for sulfur impregnation. Mater. Lett. 189, 188 (2017).
C. Li, Z. Li, Q. Li, Z. Zhang, S. Dong, and L. Yin: MOFs derived hierarchically porous TiO2 as effective chemical and physical immobilizer for sulfur species as cathodes for high-performance lithium–sulfur batteries. Electrochim. Acta 215, 689 (2016).
K. Xie, Y. Han, W. Wei, H. Yu, C. Zhang, J-G. Wang, W. Lu, and B. Wei: Fabrication of a novel TiO2/S composite cathode for high performance lithium–sulfur batteries. RSC Adv. 5 (94), 77348 (2015).
X.Z. Ma, B. Jin, H.Y. Wang, J.Z. Hou, X.B. Zhong, H.H. Wang, and P.M. Xin: S–TiO2 composite cathode materials for lithium/sulfur batteries. J. Electroanal. Chem. 736, 127 (2015).
C.J. Hart, M. Cuisinier, X. Liang, D. Kundu, A. Garsuch, and L.F. Nazar: Rational design of sulphur host materials for Li–S batteries: Correlating lithium polysulphide adsorptivity and self-discharge capacity loss. Chem. Commun. 51 (12), 2308 (2015).
Q. Li, Z. Zhang, K. Zhang, L. Xu, J. Fang, Y. Lai, and J. Li: Synthesis and electrochemical performance of TiO2–sulfur composite cathode materials for lithium–sulfur batteries. J. Solid State Electrochem. 17 (11), 2959 (2013).
J. Li, B. Ding, G. Xu, L. Hou, X. Zhang, and C. Yuan: Enhanced cycling performance and electrochemical reversibility of a novel sulfur-impregnated mesoporous hollow TiO2 sphere cathode for advanced Li–S batteries. Nanoscale 5 (13), 5743 (2013).
B. Ding, L. Shen, G. Xu, P. Nie, and X. Zhang: Encapsulating sulfur into mesoporous TiO2 host as a high performance cathode for lithium–sulfur battery. Electrochim. Acta 107, 78 (2013).
X. He, H. Hou, X. Yuan, L. Huang, J. Hu, B. Liu, J. Xu, J. Xie, J. Yang, S. Liang, and X. Wu: Electrocatalytic activity of lithium polysulfides adsorbed into porous TiO2 coated MWCNTs hybrid structure for lithium–sulfur batteries. Sci. Rep. 7, 40679 (2017).
T.A. Zegeye, C-F.J. Kuo, A.S. Wotango, C-J. Pan, H-M. Chen, A.M. Haregewoin, J-H. Cheng, W-N. Su, and B-J. Hwang: Hybrid nanostructured microporous carbon-mesoporous carbon doped titanium dioxide/sulfur composite positive electrode materials for rechargeable lithium–sulfur batteries. J. Power Sources 324, 239 (2016).
M. Yu, J. Ma, H. Song, A. Wang, F. Tian, Y. Wang, H. Qiu, and R. Wang: Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium–sulfur batteries. Energy Environ. Sci. 9 (4), 1495 (2016).
N. Moreno, Á. Caballero, J. Morales, and E. Rodríguez-Castellón: Improved performance of electrodes based on carbonized olive stones/S composites by impregnating with mesoporous TiO2 for advanced Li–S batteries. J. Power Sources 313, 21 (2016).
Y. Li, Q. Cai, L. Wang, Q. Li, X. Peng, B. Gao, K. Huo, and P.K. Chu: Mesoporous TiO2 nanocrystals/graphene as an efficient sulfur host material for high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 8 (36), 23784 (2016).
J-Y. Hwang, H.M. Kim, S-K. Lee, J-H. Lee, A. Abouimrane, M.A. Khaleel, I. Belharouak, A. Manthiram, and Y-K. Sun: High-energy, high-rate, lithium–sulfur batteries: Synergetic effect of hollow TiO2-webbed carbon nanotubes and a dual functional carbon-paper interlayer. Adv. Energy Mater. 6 (1), 1501480 (2016).
J.Q. Huang, Z. Wang, Z.L. Xu, W.G. Chong, X. Qin, X. Wang, and J.K. Kim: Three dimensional porous graphene aerogel cathode with high sulfur loading and embedded TiO2 nanoparticles for advanced lithium–sulfur batteries. ACS Appl. Mater. Interfaces 8, 28663 (2016).
L. Gao, M. Cao, Y.Q. Fu, Z. Zhong, Y. Shen, and M. Wang: Hierarchical TiO2 spheres assisted with graphene for a high performance lithium–sulfur battery. J. Mater. Chem. A 4 (42), 16454 (2016).
H. Fan, Q. Tang, X. Chen, B. Fan, S. Chen, and A. Hu: Dual-confined sulfur nanoparticles encapsulated in hollow TiO2 spheres wrapped with graphene for lithium–sulfur batteries. Chem.–Asian J. 11 (20), 2911 (2016).
Z. Zhang, Q. Li, K. Zhang, W. Chen, Y. Lai, and J. Li: Titanium-dioxide-grafted carbon paper with immobilized sulfur as a flexible free-standing cathode for superior lithium–sulfur batteries. J. Power Sources 290, 159 (2015).
Z. Zhang, Q. Li, S. Jiang, K. Zhang, Y. Lai, and J. Li: Sulfur encapsulated in a TiO2-anchored hollow carbon nanofiber hybrid nanostructure for lithium–sulfur batteries. Chem.–Eur. J. 21 (3), 1343 (2015).
Y. He, Z. Fu, Q. Zhou, M. Zhong, L. Yuan, J. Wei, X. Yang, C. Wang, and Y. Zeng: Fabrication and electrochemical behavior of a lithium–sulfur cell with a TiO2–sulfur–carbon aerogel-based cathode. Ionics 21 (11), 3065 (2015).
H. Wang, S. Li, D. Li, Z. Chen, H.K. Liu, and Z. Guo: TiO2 coated three-dimensional hierarchically ordered porous sulfur electrode for the lithium/sulfur rechargeable batteries. Energy 75, 597 (2014).
G. Liang, J. Wu, X. Qin, M. Liu, Q. Li, Y.B. He, J.K. Kim, B. Li, and F. Kang: Ultrafine TiO2 decorated carbon nanofibers as multifunctional interlayer for high-performance lithium–sulfur battery. ACS Appl. Mater. Interfaces 8 (35), 23105 (2016).
C.Y. Fan, S.Y. Liu, H.H. Li, H.F. Wang, H.C. Wang, X.L. Wu, H.Z. Sun, and J. Zhang: Synergistic design of cathode region for the high-energy-density Li–S batteries. ACS Appl. Mater. Interfaces 8, 28689 (2016).
G. Xu, J. Yuan, X. Tao, B. Ding, H. Dou, X. Yan, Y. Xiao, and X. Zhang: Absorption mechanism of carbon-nanotube paper-titanium dioxide as a multifunctional barrier material for lithium–sulfur batteries. Nano Res. 8, 3066 (2015).
Z. Xiao, Z. Yang, L. Wang, H. Nie, M. Zhong, Q. Lai, X. Xu, L. Zhang, and S. Huang: A lightweight TiO(2)/graphene interlayer, applied as a highly effective polysulfide absorbent for fast, long-life lithium–sulfur batteries. Adv. Mater. 27 (18), 2891 (2015).
H. Yao, K. Yan, W. Li, G. Zheng, D. Kong, Z.W. Seh, V.K. Narasimhan, Z. Liang, and Y. Cui: Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 7 (10), 3381 (2014).
G. Zhou, Y. Zhao, C. Zu, and A. Manthiram: Free-standing TiO2 nanowire–embedded graphene hybrid membrane for advanced Li/dissolved polysulfide batteries. Nano Energy 12, 240 (2015).
F. Li, G. Wang, P. Wang, J. Yang, K. Zhang, Y. Liu, and Y. Lai: High-performance lithium–sulfur batteries with a carbonized bacterial cellulose/TiO2 modified separator. J. Electroanal. Chem. 788, 150 (2017).
H. Wei, E.F. Rodriguez, A.S. Best, A.F. Hollenkamp, D. Chen, and R.A. Caruso: Chemical bonding and physical trapping of sulfur in mesoporous Magnéli Ti4O7 microspheres for high-performance Li–S battery. Adv. Energy Mater. 7 (4), 1601616 (2017).
X. Tao, J. Wang, Z. Ying, Q. Cai, G. Zheng, Y. Gan, H. Huang, Y. Xia, C. Liang, W. Zhang, and Y. Cui: Strong sulfur binding with conducting Magnéli-phase TinO2 n−1 nanomaterials for improving lithium–sulfur batteries. Nano Lett. 14 (9), 5288 (2014).
Q. Pang, D. Kundu, M. Cuisinier, and L.F. Nazar: Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium–sulphur batteries. Nat. Commun. 5, 4759 (2014).
L. Ni, Z. Wu, G. Zhao, C. Sun, C. Zhou, X. Gong, and G. Diao: Core–shell structure and interaction mechanism of gamma-MnO2 coated sulfur for improved lithium–sulfur batteries. Small 13, 1603466–1603476 (2017).
X. Liang and L.F. Nazar: In situ reactive assembly of scalable core–shell sulfur–MnO2 composite cathodes. ACS Nano 10 (4), 4192 (2016).
X. Liang, C. Hart, Q. Pang, A. Garsuch, T. Weiss, and L.F. Nazar: A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 6, 5682 (2015).
H. Xu, L. Qie, and A. Manthiram: An integrally-designed, flexible polysulfide host for high-performance lithium–sulfur batteries with stabilized lithium–metal anode. Nano Energy 26, 224 (2016).
Y. Li, D. Ye, W. Liu, B. Shi, R. Guo, H. Zhao, H. Pei, J. Xu, and J. Xie: A MnO2/graphene oxide/multi-walled carbon nanotubes–sulfur composite with dual-efficient polysulfide adsorption for improving lithium–sulfur batteries. ACS Appl. Mater. Interfaces 8, 28566 (2016).
Z. Li, J. Zhang, and X.W. Lou: Hollow carbon nanofibers filled with MnO2 nanosheets as efficient sulfur hosts for lithium–sulfur batteries. Angew. Chem., Int. Ed. 54 (44), 12886 (2015).
S. Rehman, T. Tang, Z. Ali, X. Huang, and Y. Hou: Integrated design of MnO2@carbon hollow nanoboxes to synergistically encapsulate polysulfides for empowering lithium sulfur batteries. Small 13 (20), 1700087 (2017).
W. Sun, X. Ou, X. Yue, Y. Yang, Z. Wang, D. Rooney, and K. Sun: A simply effective double-coating cathode with MnO2 nanosheets/graphene as functionalized interlayer for high performance lithium–sulfur batteries. Electrochim. Acta 207, 198 (2016).
Y. Guo, G. Zhao, N. Wu, Y. Zhang, M. Xiang, B. Wang, H. Liu, and H. Wu: Efficient synthesis of graphene nanoscrolls for fabricating sulfur-loaded cathode and flexible hybrid interlayer toward high-performance Li–S batteries. ACS Appl. Mater. Interfaces 8 (50), 34185 (2016).
T. An, D. Deng, M. Lei, Q-H. Wu, Z. Tian, M. Zheng, and Q. Dong: MnO modified carbon nanotubes as a sulfur host with enhanced performance in Li/S batteries. J. Mater. Chem. A 4 (33), 12858 (2016).
J. Liu, F. Liu, K. Gao, J. Wu, and D. Xue: Recent developments in the chemical synthesis of inorganic porous capsules. J. Am. Chem. Soc. 19 (34), 6073 (2009).
M. Liu, Q. Li, X. Qin, G. Liang, W. Han, D. Zhou, Y.B. He, B. Li, and F. Kang: Suppressing self-discharge and shuttle effect of lithium–sulfur batteries with V2O5-decorated carbon nanofiber interlayer. Small 13 (12), 1602539–1602545 (2017).
W. Li, J. Hicks-Garner, J. Wang, J. Liu, A.F. Gross, E. Sherman, J. Graetz, J.J. Vajo, and P. Liu: V2O5 polysulfide anion barrier for long-lived Li–S batteries. Chem. Mater. 26 (11), 3403 (2014).
Y. Zhang, X. Wu, H. Feng, L. Wang, A. Zhang, T. Xia, and H. Dong: Effect of nanosized Mg0.8Cu0.2O on electrochemical properties of Li/S rechargeable batteries. Int. J. Hydrogen Energy 34 (3), 1556 (2009).
Y. Zhang, L. Wang, A. Zhang, Y. Song, X. Li, H. Feng, X. Wu, and P. Du: Novel V2O5/S composite cathode material for the advanced secondary lithium batteries. Solid State Ionics 181 (17–18), 835 (2010).
M-S. Kim, E.S. Shin, J-S. Kim, W.I. Cho, and S.H. Oh: The effect of V2O5/C additive on the suppression of polysulfide dissolution in Li–sulfur batteries. J. Electroceram. 33 (3–4), 142 (2014).
K.T. Lee, R. Black, T. Yim, X. Ji, and L.F. Nazar: Surface-initiated growth of thin oxide coatings for Li–sulfur battery cathodes. Adv. Energy Mater. 2 (12), 1490 (2012).
Y-S. Su and A. Manthiram: Sulfur/lithium-insertion compound composite cathodes for Li–S batteries. J. Power Sources 270, 101 (2014).
L.P. Zhang, Y.F. Wang, S.Q. Gou, and J.H. Zeng: All inorganic frameworks of Tin dioxide shell as cathode material for lithium sulfur batteries with improved cycle performance. J. Phys. Chem. C 119 (52), 28721 (2015).
B. Cao, D. Li, B. Hou, Y. Mo, L. Yin, and Y. Chen: Synthesis of double-shell SnO2@C hollow nanospheres as sulfur/sulfide cages for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 8, 27795–27802 (2016).
A.Y. Kim, M.K. Kim, J.Y. Kim, Y. Wen, L. Gu, V-D. Dao, H-S. Choi, D. Byun, and J.K. Lee: Ordered SnO nanoparticles in MWCNT as a functional host material for high-rate lithium–sulfur battery cathode. Nano Res. 10, 2083 (2017).
Y.J. Choi, B.S. Jung, D.J. Lee, J.H. Jeong, K.W. Kim, H.J. Ahn, K.K. Cho, and H.B. Gu: Electrochemical properties of sulfur electrode containing nano Al2O3 for lithium/sulfur cell. Phys. Scr. T129, 62 (2007).
K. Dong, S. Wang, H. Zhang, and J. Wu: Preparation and electrochemical performance of sulfur–alumina cathode material for lithium–sulfur batteries. Mater. Res. Bull. 48 (6), 2079 (2013).
X. Han, Y. Xu, X. Chen, Y-C. Chen, N. Weadock, J. Wan, H. Zhu, Y. Liu, H. Li, G. Rubloff, C. Wang, and L. Hu: Reactivation of dissolved polysulfides in Li–S batteries based on atomic layer deposition of Al2O3 in nanoporous carbon cloth. Nano Energy 2 (6), 1197 (2013).
X. Li, J. Liu, B. Wang, M.N. Banis, B. Xiao, R. Li, T-K. Sham, and X. Sun: Nanoscale stabilization of Li–sulfur batteries by atomic layer deposited Al2O3. RSC Adv. 4 (52), 27126 (2014).
M. Yu, W. Yuan, C. Li, J-D. Hong, and G. Shi: Performance enhancement of a graphene–sulfur composite as a lithium–sulfur battery electrode by coating with an ultrathin Al2O3 film via atomic layer deposition. J. Mater. Chem. A 2 (20), 7360 (2014).
Z. Zhang, Y. Lai, Z. Zhang, K. Zhang, and J. Li: Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochim. Acta 129, 55 (2014).
R. Song, R. Fang, L. Wen, Y. Shi, S. Wang, and F. Li: A trilayer separator with dual function for high performance lithium–sulfur batteries. J. Power Sources 301, 179 (2016).
F. Li, L. Yang, G. Xu, H. Xiaoqiang, X. Yang, X. Wei, Z. Ren, G. Shen, and G. Han: Hydrothermal self-assembly of hierarchical flower-like ZnO nanospheres with nanosheets and their application in Li-ion batteries. J. Alloys Compd. 577, 663 (2013).
T. Zhao, Y. Ye, X. Peng, G. Divitini, H-K. Kim, C-Y. Lao, P.R. Coxon, K. Xi, Y. Liu, C. Ducati, R. Chen, and R.V. Kumar: Advanced lithium–sulfur batteries enabled by a bio-inspired polysulfide adsorptive brush. Adv. Funct. Mater. 26 (46), 8418 (2016).
M. Yu, A. Wang, F. Tian, H. Song, Y. Wang, C. Li, J.D. Hong, and G. Shi: Dual-protection of a graphene–sulfur composite by a compact graphene skin and an atomic layer deposited oxide coating for a lithium–sulfur battery. Nanoscale 7 (12), 5292 (2015).
X. Liang, Q. Song, Y. Liu, and H. Liu: Preparation of ZnO porous nanostructures and its application in cathode material for lithium sulfur battery. Int. J. Electrochem. Sci. 10, 9333 (2015).
H. Tang, S. Yao, M. Jing, X. Wu, J. Hou, X. Qian, D. Rao, X. Shen, X. Xi, and K. Xiao: Mg0.6Ni0.4O hollow nanofibers prepared by electrospinning as additive for improving electrochemical performance of lithium–sulfur batteries. J. Alloys Compd. 650, 351 (2015).
Y. Zhang, Y. Zhao, A. Yermukhambetova, Z. Bakenov, and P. Chen: Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 1 (2), 295 (2013).
M-S. Song, S-C. Han, H-S. Kim, J-H. Kim, K-T. Kim, Y-M. Kang, H-J. Ahn, S.X. Dou, and J-Y. Lee: Effects of nanosized adsorbing material on electrochemical properties of sulfur cathodes for Li/S secondary batteries. J. Electrochem. Soc. 151 (6), A791 (2004).
Y. Zhao, M. Liu, W. Lv, Y-B. He, C. Wang, Q. Yun, B. Li, F. Kang, and Q-H. Yang: Dense coating of Li4Ti5O12 and graphene mixture on the separator to produce long cycle life of lithium–sulfur battery. Nano Energy 30, 1 (2016).
K. Xie, Y. You, K. Yuan, W. Lu, K. Zhang, F. Xu, M. Ye, S. Ke, C. Shen, X. Zeng, X. Fan, and B. Wei: Ferroelectric-enhanced polysulfide trapping for lithium–sulfur battery improvement. Adv. Mater. 29 (6), 1604724–1604729 (2017).
C.S. Kim, A. Guerfi, P. Hovington, J. Trottier, C. Gagnon, F. Barray, A. Vijh, M. Armand, and K. Zaghib: Facile dry synthesis of sulfur–LiFePO4 core–shell composite for the scalable fabrication of lithium/sulfur batteries. Electrochem. Commun. 32, 35 (2013).
Q. Fan, W. Liu, Z. Weng, Y. Sun, and H. Wang: Ternary hybrid material for high-performance lithium–sulfur battery. J. Am. Chem. Soc. 137 (40), 12946 (2015).
H. Wang, T. Zhou, D. Li, H. Gao, G. Gao, A. Du, H. Liu, and Z. Guo: Ultrathin cobaltosic oxide nanosheets as an effective sulfur encapsulation matrix with strong affinity toward polysulfides. ACS Appl. Mater. Interfaces 9 (5), 4320 (2017).
H. Cheng, S. Wang, D. Tao, and M. Wang: Sulfur/Co3O4 nanotube composite with high performances as cathode materials for lithium sulfur batteries. Funct. Mater. Lett. 07 (03), 1450020 (2014).
C. Wan, W. Wu, C. Wu, J. Xu, and L. Guan: A layered porous ZrO2/RGO composite as sulfur host for lithium–sulfur batteries. RSC Adv. 5 (7), 5102 (2015).
Y. Zhou, C. Zhou, Q. Li, C. Yan, B. Han, K. Xia, Q. Gao, and J. Wu: Enabling prominent high-rate and cycle performances in one lithium–sulfur battery: Designing permselective gateways for Li+ transportation in holey-CNT/S cathodes. Adv. Mater. 27 (25), 3774 (2015).
Q. Qu, T. Gao, H. Zheng, Y. Wang, X. Li, X. Li, J. Chen, Y. Han, J. Shao, and H. Zheng: Strong surface-bound sulfur in conductive MoO2 matrix for enhancing Li–S battery performance. Adv. Mater. Interfaces 2 (7), 1500048 (2015).
Z. Ma, Q. Liu, and S. Wang: Sulfur–graphene composite with molybdenum particles for stabilizing lithium–sulfur batteries. RSC Adv. 5 (3), 2096 (2015).
Y. Tao, Y. Wei, J. Wang, Y. Liu, W. Qiao, L. Ling, and D. Long: Kinetically-enhanced polysulfide redox reactions by Nb2O5 nanocrystal for high-rate lithium–sulfur battery. Energy Environ. Sci. 9, 3230 (2016).
W. Zhang, C. Lin, S. Cong, J. Hou, B. Liu, F. Geng, J. Jin, M. Wu, and Z. Zhao: W18O49 nanowire composites as novel barrier layers for Li–S batteries based on high loading of commercial micro-sized sulfur. RSC Adv. 6 (18), 15234 (2016).
R. Ponraj, A.G. Kannan, J.H. Ahn, and D.W. Kim: Improvement of cycling performance of lithium–sulfur batteries by using magnesium oxide as a functional additive for trapping lithium polysulfide. ACS Appl. Mater. Interfaces 8 (6), 4000 (2016).
G-Q. Ma, Z-Y. Wen, Q-S. Wang, J. Jin, X-W. Wu, and J-C. Zhang: Effects of CeO2 nano-crystal on electrochemical properties of lithium/sulfur batteries. J. Inorg. Mater. 30 (9), 913 (2015).
C. Zhao, C. Shen, F. Xin, Z. Sun, and W. Han: Prussian blue-derived Fe2O3/sulfur composite cathode for lithium–sulfur batteries. Mater. Lett. 137, 52 (2014).
F. Sun, J. Wang, D. Long, W. Qiao, L. Ling, C. Lv, and R. Cai: A high-rate lithium–sulfur battery assisted by nitrogen-enriched mesoporous carbons decorated with ultrafine La2O3 nanoparticles. J. Mater. Chem. A 1 (42), 13283 (2013).
H. Yao, G. Zheng, P.C. Hsu, D. Kong, J.J. Cha, W. Li, Z.W. Seh, M.T. McDowell, K. Yan, Z. Liang, V.K. Narasimhan, and Y. Cui: Improving lithium–sulphur batteries through spatial control of sulphur species deposition on a hybrid electrode surface. Nat. Commun. 5, 3943 (2014).
J. Jiang, J. Zhu, W. Ai, X. Wang, Y. Wang, C. Zou, W. Huang, and T. Yu: Encapsulation of sulfur with thin-layered nickel-based hydroxides for long-cyclic lithium–sulfur cells. Nat. Commun. 6, 8622 (2015).
X-Q. Niu, X-L. Wang, D-H. Wang, Y. Li, Y-J. Zhang, Y-D. Zhang, T. Yang, T. Yu, and J-P. Tu: Metal hydroxide—A new stabilizer for the construction of sulfur/carbon composites as high-performance cathode materials for lithium–sulfur batteries. J. Mater. Chem. A 3 (33), 17106 (2015).
J. Zhang, H. Hu, Z. Li, and X.W. Lou: Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium–sulfur batteries. Angew. Chem., Int. Ed. 55 (12), 3982 (2016).
X-Q. Niu, X-L. Wang, D. Xie, D-H. Wang, Y-D. Zhang, Y. Li, T. Yu, and J-P. Tu: Nickel hydroxide-modified sulfur/carbon composite as a high-performance cathode material for lithium sulfur battery. ACS Appl. Mater. Interfaces 7 (30), 16715 (2015).
A. Garsuch, S. Herzog, L. Montag, A. Krebs, and K. Leitner: Performance of blended TiS2/sulfur/carbon cathodes in lithium–sulfur cells. ECS Electrochem. Lett. 1, A24 (2012).
Z. Ma, Z. Li, K. Hu, D. Liu, J. Huo, and S. Wang: The enhancement of polysulfide absorption in LiS batteries by hierarchically porous CoS2/carbon paper interlayer. J. Power Sources 325, 71 (2016).
S. Zhang, X. Yu, H. Yu, Y. Chen, P. Gao, C. Li, and C. Zhu: Growth of ultrathin MoS2 nanosheets with expanded spacing of (002) plane on carbon nanotubes for high-performance sodium-ion battery anodes. ACS Appl. Mater. Interfaces 6 (24), 21880 (2014).
X. Li, K. Zhao, L. Zhang, Z. Ding, and K. Hu: MoS2-decorated coaxial nanocable carbon aerogel composites as cathode materials for high performance lithium–sulfur batteries. J. Alloys Compd. 692, 40 (2017).
E.P. Kamphaus and P.B. Balbuena: Long-chain polysulfide retention at the cathode of Li–S batteries. J. Phys. Chem. C 120 (8), 4296 (2016).
H. Wang, Q. Zhang, H. Yao, Z. Liang, H.W. Lee, P.C. Hsu, G. Zheng, and Y. Cui: High electrochemical selectivity of edge versus terrace sites in two-dimensional layered MoS2 materials. Nano Lett. 14 (12), 7138 (2014).
P.T. Dirlam, J. Park, A.G. Simmonds, K. Domanik, C.B. Arrington, J.L. Schaefer, V.P. Oleshko, T.S. Kleine, K. Char, R.S. Glass, C.L. Soles, C. Kim, N. Pinna, Y.E. Sung, and J. Pyun: Elemental sulfur and molybdenum disulfide composites for Li–S batteries with long cycle life and high-rate capability. ACS Appl. Mater. Interfaces 8 (21), 13437 (2016).
X. Li, Y. Lu, Z. Hou, W. Zhang, Y. Zhu, Y. Qian, J. Liang, and Y. Qian: SnS2-compared to SnO2-stabilized S/C composites toward high-performance lithium sulfur batteries. ACS Appl. Mater. Interfaces 8 (30), 19550 (2016).
X. Li, L. Chu, Y. Wang, and L. Pan: Anchoring function for polysulfide ions of ultrasmall SnS2 in hollow carbon nanospheres for high performance lithium–sulfur batteries. Mater. Sci. Eng., B 205, 46 (2016).
T.Y. Lei, W. Chen, J.W. Huang, C.Y. Yan, H.X. Sun, C. Wang, W.L. Zhang, Y.R. Li, and J. Xiong: Multi-functional layered WS2 nanosheets for enhancing the performance of lithium–sulfur batteries. Adv. Energy Mater. 7 (4), 1601843 (2017).
J.D. Liu, X.S. Zheng, Z.F. Shi, and S.Q. Zhang: Sulfur/mesoporous carbon composites combined with γ-MnS as cathode materials for lithium/sulfur batteries. Ionics 20 (5), 659 (2013).
S.S. Zhang and D.T. Tran: Pyrite FeS2 as an efficient adsorbent of lithium polysulphide for improved lithium–sulphur batteries. J. Mater. Chem. A 4 (12), 4371 (2016).
Y. Lu, X. Li, J. Liang, L. Hu, Y. Zhu, and Y. Qian: A simple melting-diffusing-reacting strategy to fabricate S/NiS2–C for lithium–sulfur batteries. Nanoscale 8 (40), 17616 (2016).
K. Sun, D. Su, Q. Zhang, D.C. Bock, A.C. Marschilok, K.J. Takeuchi, E.S. Takeuchi, and H. Gan: Interaction of CuS and sulfur in Li–S battery system. J. Electrochem. Soc. 162, A2834 (2015).
X. Gao, Y. Wang, Z. Ma, W. Jiang, Y. Zou, and C. Lu: A ternary sulphonium composite Cu3BiS3/S as cathode materials for lithium–sulfur batteries. J. Mater. Sci. 51 (11), 5139 (2016).
J-Q. Huang, B. Zhang, Z-L. Xu, S. Abouali, M. Akbari Garakani, J. Huang, and J-K. Kim: Novel interlayer made from Fe3C/carbon nanofiber webs for high performance lithium–sulfur batteries. J. Power Sources 285, 43 (2015).
E.S. Sim, G.S. Yi, M. Je, Y. Lee, and Y-C. Chung: Understanding the anchoring behavior of titanium carbide-based MXenes depending on the functional group in Li–S batteries: A density functional theory study. J. Power Sources 342, 64 (2017).
X. Zhao, M. Liu, Y. Chen, B. Hou, N. Zhang, B. Chen, N. Yang, K. Chen, J. Li, and L. An: Fabrication of layered Ti3C2 with an accordion-like structure as a potential cathode material for high performance lithium–sulfur batteries. J. Mater. Chem. A 3 (15), 7870 (2015).
H.J. Peng, G. Zhang, X. Chen, Z.W. Zhang, W.T. Xu, J.Q. Huang, and Q. Zhang: Enhanced electrochemical kinetics on conductive polar mediators for lithium–sulfur batteries. Angew. Chem., Int. Ed. 55 (42), 12990 (2016).
Z. Sun, J. Zhang, L. Yin, G. Hu, R. Fang, H.M. Cheng, and F. Li: Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium–sulfur batteries. Nat. Commun. 8, 14627 (2017).
N. Mosavati, S.O. Salley, and K.Y.S. Ng: Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries. J. Power Sources 340, 210 (2017).
J. Zhou, R. Li, X. Fan, Y. Chen, R. Han, W. Li, J. Zheng, B. Wang, and X. Li: Rational design of a metal–organic framework host for sulfur storage in fast, long-cycle Li–S batteries. Energy Environ. Sci. 7 (8), 2715 (2014).
W. Bao, Z. Zhang, Y. Qu, C. Zhou, X. Wang, and J. Li: Confine sulfur in mesoporous metal–organic framework@reduced graphene oxide for lithium sulfur battery. J. Alloys Compd. 582, 334 (2014).
R. Demir-Cakan, M. Morcrette, F. Nouar, C. Davoisne, T. Devic, D. Gonbeau, R. Dominko, C. Serre, G. Ferey, and J.M. Tarascon: Cathode composites for Li–S batteries via the use of oxygenated porous architectures. J. Am. Chem. Soc. 133 (40), 16154 (2011).
Y. Hou, H. Mao, and L. Xu: MIL-100(V) and MIL-100(V)/rGO with various valence states of vanadium ions as sulfur cathode hosts for lithium–sulfur batteries. Nano Res. 10 (1), 344 (2016).
L. Bai, D. Chao, P. Xing, L.J. Tou, Z. Chen, A. Jana, Z.X. Shen, and Y. Zhao: Refined sulfur nanoparticles immobilized in metal-organic polyhedron as stable cathodes for Li–S battery. ACS Appl. Mater. Interfaces 8 (23), 14328 (2016).
Z. Zhao, S. Wang, R. Liang, Z. Li, Z. Shi, and G. Chen: Graphene-wrapped chromium-MOF(MIL-101)/sulfur composite for performance improvement of high-rate rechargeable Li–S batteries. J. Mater. Chem. A 2 (33), 13509 (2014).
X. Liang, C.Y. Kwok, F. Lodi-Marzano, Q. Pang, M. Cuisinier, H. Huang, C.J. Hart, D. Houtarde, K. Kaup, H. Sommer, T. Brezesinski, J. Janek, and L.F. Nazar: Tuning transition metal oxide–sulfur interactions for long life lithium sulfur batteries: The “Goldilocks” principle. Adv. Energy Mater. 6 (6), 1501636 (2016).
M. Hagen, D. Hanselmann, K. Ahlbrecht, R. Maça, D. Gerber, and J. Tübke: Lithium–sulfur cells: The gap between the state-of-the-art and the requirements for high energy battery cells. Adv. Energy Mater. 5 (16), 1401986 (2015).
D. Lv, J. Zheng, Q. Li, X. Xie, S. Ferrara, Z. Nie, L.B. Mehdi, N.D. Browning, J-G. Zhang, G.L. Graff, J. Liu, and J. Xiao: High energy density lithium–sulfur batteries: Challenges of thick sulfur cathodes. Adv. Energy Mater. 5 (16), 1402290 (2015).
R. Fang, S. Zhao, P. Hou, M. Cheng, S. Wang, H.M. Cheng, C. Liu, and F. Li: 3D interconnected electrode materials with ultrahigh areal sulfur loading for Li–S batteries. Adv. Mater. 28 (17), 3374 (2016).
L. Miao, W. Wang, K. Yuan, Y. Yang, and A. Wang: A lithium–sulfur cathode with high sulfur loading and high capacity per area: A binder-free carbon fiber cloth-sulfur material. Chem. Commun. 50 (87), 13231 (2014).
R. Fang, S. Zhao, S. Pei, X. Qian, P.X. Hou, H.M. Cheng, C. Liu, and F. Li: Toward more reliable lithium–sulfur batteries: An all-graphene cathode structure. ACS Nano 10 (9), 8676 (2016).
ACKNOWLEDGMENTS
This work has been supported by the National Natural Science Foundation of China (Nos. 51572116 and 51202094); the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gu, X., Lai, C. Recent development of metal compound applications in lithium–sulphur batteries. Journal of Materials Research 33, 16–31 (2018). https://doi.org/10.1557/jmr.2017.282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2017.282