Abstract
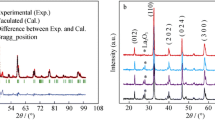
In this paper, we report the structural, electrical, and magnetic properties of polycrystalline La0.85-xSmxNa0.15MnO3 (x = 0.05, 0.1, 0.15) manganites. Rietveld refinement of x-ray data infers that doped manganite compounds possess a rhombohedrally distorted structure (space group \(R\overline{3}C\)). Both lattice parameter and unit cell volume decrease and a systematic change in both Mn-O-Mn bond angle and tolerance factor is observed with Sm content. Resistivity measurements discern metal-insulator transition (TP). For x = 0.15 sample, a double metal-insulator transition with a single ferromagnetic transition is depicted. All samples exhibit extrinsic magnetoresistance (MR) effect. A large value of MR of 65% (253 K, 8 T) is associated with grain and grain boundary formation. The highest low-field MR of 23% (12 K, 2 T) and 35.2% (23 K, 2 T) for x = 0.05 and 0.1 is observed. The electronic and magnetic inhomogeneities induced by Sm and nonmagnetic metal Na phases account for MR properties.









Similar content being viewed by others
References
M.B. Salamon and M. Jaime: The physics of manganites: Structure and transport. Rev. Mod. Phys. 73, 583 (2001).
G.H. Rao, J.R. Sun, K. Barner, and N. Hamad: Crystal structure and magnetoresistance of Na-doped LaMnO3. J. Phys.: Condens. Matter 11, 1523 (1999).
S.L. Ye, W.H. Song, J.M. Dai, K.Y. Wang, S.G. Wang, J.J. Du, Y.P. Sun, J. Fang, J.L. Chen, and B.J. Gao: Large room-temperature magnetoresistance and phase separation in La1−xNaxMnO3 with 0.1≤x≤ 0.3. J. Appl. Phys. 90, 2943 (2001).
S. Asthana, D. Bahadur, A. Nigam, and S.K. Malik: Magneto-transport studies on (Pr1/3Sm2/3)2/3A1/3MnO3 (A = Ca, Sr and Ba) compounds. J. Phys.: Condens. Matter 16, 5297 (2004).
J. Fontcuberta, B. Martınez, A. Seffar, S. Pinol, J.L. Garcia-Munoz, and X. Obradors: Colossal magnetoresistance of ferromagnetic manganites: Structural tuning and mechanisms. Phys. Rev. Lett. 76, 1122 (1996).
A.I. Tovstolytkin, A.M. Pogorily, D.I. Podyalovskii, V.M. Kalita, A.F. Lozenko, P.O. Trotsenko, S.M. Ryabchenko, A.G. Belous, O.I. Vunov, and O.Z. Yanchevskii: Vacancy-induced enhancement of magnetic interactions in (Ca, Na)-doped lanthanum manganites. J. Appl. Phys. 102, 063902 (2007).
S. Qixiang, W. Guiying, Y. Guoqing, M. Qiang, W. Wenqi, and P. Zhensheng: Influence of the substitution of Sm, Gd, and Dy for La in La0.7Sr0.3MnO3 on its magnetic and electric properties and strengthening effect on room-temperature CMR. J. Rare Earths 26, 821 (2008).
Z.M. Wang, G. Ni, H. Sanga, and Y.W. Du: The effect of average A-site cation radius on TC in perovskite manganites. J. Magn. Magn. Mater. 234, 213 (2001).
R.D. Shannon: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., A 32, 751 (1976).
J. Rodriguez-Carvajal: FULLPROF Version 3.0.0 (Laboratoire Leon Brillouin, CEA–CNRS, 1995).
D. Varshney, N. Dodiya, and M.W. Shaikh: Structural properties and electrical resistivity of Na-substituted lanthanum manganites: La1-xNaxMnO3 (x = 0.1, 0.125 and 0.15). J. Alloys Compd. 509, 7447 (2011).
R. Mahendiran, S.K. Tiwary, A.K. Raychaudhuri, and T.V. Ramakrishnan: Structure, electron-transport properties, and giant magnetoresistance of hole-doped LaMnO3 systems. Phys. Rev. B 53, 3348 (1996).
S. Roy, Y.Q. Guo, S. Venkatesh, and N. Ali: Interplay of structure and transport properties of sodium-doped lanthanum manganite. J. Phys.: Condens. Matter 13, 9547 (2001).
Y. Kalyana Lakshmia, G. Venkataiaha, M. Vithalb, and P. Venugopal Reddy: Magnetic and electrical behavior of La1−xAxMnO3 (A = Li, Na, K and Rb) manganites. Physica B 403, 3059 (2008).
J. Vergara, R.J. Ortega-Hertogs, V. Madurga, F. Sapina, Z. El-Fadli, E. Martinez, A. Beltran, and K.V. Rao: Effect of disorder produced by cationic vacancies at the B sites on the electronic properties of mixed valence manganites. Phys. Rev. B 60, 1127 (1999).
M. Mazaheri and M. Akhavan: Electrical behavior of nano-polycrystalline (La1−y Ky)0.7Ba0.3MnO3 manganites. J. Magn. Magn. Mater. 322, 3255 (2010).
G. Venkataiah, Y. Kalyana Lakshmi, and P. Venugopal Reddy: Thermopower studies of Pr0.67D0.33MnO3 manganite system. J. Phys. D: Appl. Phys. 40, 721 (2007).
V.P.S. Awana, R. Tripathi, N. Kumar, H. Kishan, G.L. Bhalla, R. Zeng, L.S. Sharth Chandra, V. Ganesan, and H.U. Habermeier: Magnetotransport of La0.7Ca0.3−xSrxMnO3 (Ag): A potential room temperature bolometer and magnetic sensor. J. Appl. Phys. 107, 09D723 (2010).
V.P.S. Awana, R. Tripathi, S. Balamurugan, H. Kishan, and E. Takayama-Muromachi: Magneto-transport of high TCR (temperature coefficient of resistance) La2/3Ca1/3MnO3: Ag polycrystalline composites. Solid State Commun. 40, 410–415 (2006).
N. Dodiya and D. Varshney: Structural properties and Raman spectroscopy of rhombohedral La1−xNaxMnO3 with (0.075 ≤ x ≤ 0.15). J. Mol. Struct. 1031, 104 (2013).
D. Wu, Y. Deng, C.L. Mak, K.H. Wong, A.D. Li, M.S. Zhang, and N.B. Min: Raman scattering study of La-, Nd and Sm-substituted Bi4Ti3O12. Appl. Phys. A: Mater. Sci. Process 80, 607 (2005).
A. Urushibara, Y. Moritomo, T. Arima, A. Asamitsu, G. Kido, and Y. Tokura: Insulator-metal transition and giant magnetoresistance in La1−xSrxMnO3. Phys. Rev. B 51, 14103 (1995).
P. Fulde and J. Jensen: Electronic heat capacity of the rare-earth metals. Phys. Rev. B 27, 4085 (1983).
K. Kubo and N. Ohata: A quantum theory of double exchange. J. Phys. Soc. Jpn. 33, 21 (1972).
P. Schiffer, A.P. Ramirez, W. Bao, and S.W. Cheong: Low temperature magnetoresistance and the magnetic phase diagram of La1−xCaxMnO3. Phys. Rev. Lett. 75, 3336 (1995).
Y. Kalyana Lakshmi, G. Venkataiah, and P. Venugopal Reddy: Magnetoelectric behavior of sodium doped lanthanum manganites. J. Appl. Phys. 106, 023707 (2009).
L. Ghivelder, I.A. Castillo, N. McN Alford, G.J. Tomka, P.C. Riedi, J. MacManus-Driscoll, A.K.M. Akther Hossain, and L.F. Cohen: Specific heat of La1−xCaxMnO3−δ. J. Magn. Magn. Mater. 189, 274 (1998).
D. Varshney and N. Dodiya: Electrical resistivity of alkali metal doped manganites LaxAyMnwO3 (A=Na, K, Rb): Role of electron-phonon, electron-electron and electron-magnon interactions. Curr. Appl. Phys. 13, 1188–1198 (2013).
M. Quijada, J. Cerne, J.R. Simpson, H.D. Drew, K.H. Ahn, A.J. Millis, R. Shreekala, R. Ramesh, M. Rajeswari, and T. Venkatesan: Optical conductivity of manganites: Crossover from Jahn-Teller small polaron to coherent transport in the ferromagnetic state. Phys. Rev. B 58, 16093 (1998).
L. Malavasi, C. Ritter, M.C. Mozzati, C. Tealdi, M. Saiful Islam, C. Bruno Azzoni, and G. Flor: Effects of cation vacancy distribution in doped LaMnO3+δ perovskites. J. Solid State Chem. 178, 2042 (2005).
M. Egilmez, K.H. Chow, J. Jung, I. Fan, A.I. Mansour, and Z. Salman: Metal-insulator transition, specific heat, and grain-boundary-induced disorder in Sm0.55Sr0.45MnO3. Appl. Phys. Lett. 92, 132505 (2008).
N. Mannella, W.L. Yang, K. Tanaka, X.J. Zhou, H. Zheng, J.F. Mitchell, J. Zaanen, T.P. Devereaux, N. Nagaosa, Z. Hussain, and Z.X. Shen: Polaron coherence condensation as the mechanism for colossal magnetoresistance in layered manganites. Phys. Rev. B 76, 233102 (2007).
N. Mannella, W.L. Yang, X.J. Zhou, H. Zheng, J.F. Mitchell, J. Zaanen, T.P. Devereaux, N. Nagaosa, Z. Hussain, and Z.X. Shen: Nodal quasiparticle in pseudogapped colossal magnetoresistive manganites. Nature 438, 474 (2005).
A.S. Alexandrov and N.F. Mott: Polarons and Bipolarons (World Scientific, Singapore, 1995).
G.J. Snyder, R. Hiskes, S. DiCarolis, M.R. Beasley, and T.H. Geballe: Intrinsic electrical transport and magnetic properties of La0.67Ca0.33MnO3 and La0.67Sr0.33MnO3 MOCVD thin films and bulk material. Phys. Rev. B 53, 14434 (1996).
M.P. Tosi: Of ionic solids in the Born model. Solid State Phys. 16, 1 (1964).
D.W. Hafemeister and W.H. Flygare: Outer-shell overlap integral as a function of distance for halogen-halogen, halogen-alkali, and alkali-alkali ions in the alkali halide lattices. J. Chem. Phys. 43, 795 (1965).
J.C. Slater and J.G. Kirkwood: The van der Waals forces in gases. Phys. Rev. 37, 682 (1931).
V. Markovich, I. Fita, R. Puzniak, E. Rozenberg, C. Martin, A. Wisniewski, A. Maignan, B. Raveau, Y. Yuzhelevskii, and G. Gorodetsky: Effect of pressure on magnetic and transport properties of CaMn1−xRuxO3 (x = 0–0.15): Collapse of ferromagnetic phase in CaMn0.9Ru0.1O3. Phys. Rev. B 70, 024403 (2004).
D. Varshney, N. Dodiya: Interpretation of metallic and semiconducting temperature dependent resistivity of La0.91Rb0.06Mn0.94O3 manganites. Solid State Sci. 13, 1623–1632 (2011).
D. Varshney, N. Dodiya: Electrical resistivity of the hole doped La0.8Sr0.2MnO3 manganites: Role of electron–electron/phonon/magnon interactions. Mater. Chem. Phys. 129, 896–904 (2011).
N. Marzari and D. Vanderbilti: Maximally localized generalized Wannier functions for composite energy bands. Phys. Rev. B 56, 12847 (1997).
A.J. Millis: Cooperative Jahn-Teller effect and electron-phonon coupling in La1−xAx MnO3. Phys. Rev. B 53, 8434 (1996).
ACKNOWLEDGMENTS
Financial assistance from UGC, New Delhi, India is gratefully acknowledged. Authors are thankful to UGC-DAE CSR, Indore for providing characterization facilities. Useful discussions with Dr. R. Rawat are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
APPENDIX
APPENDIX
The understanding of the dynamical properties of materials requires the formulation of an effective interionic potential and is of substantial importance, as there is much disagreement as to whether long- or short-range interactions are at the origin of the properties of doped manganites. While developing a specific model, we assume that the change in force constants is small; the short-range interactions are effective up to the second-neighbor ions; and the atoms are on hold by elastic forces in the harmonic approximation without any internal strains within the crystal. The crystal energy for a particular lattice separation (r) as:
The first term is the Coulomb energy, and follows:
Here, αm as the Madelung constant36 and rij being the separation distance between i and j ions.
The short-range overlap repulsive energy is the second term in Eq. (A1) as:
following Hafemeister and Flygare.37 The notations ri (rj) refer to the ionic radii, k is the structure factor, n (n′) is the number of nearest (next nearest) ions, respectively. Further, the notations b and ρ denote the hardness and range parameters, respectively.
The Pauling coefficients, βij are defined in terms of valence [Zi (Zj)] and number of the outermost electrons [ni (nj)] in the anions (cations), respectively, as:
The last term in Eq. (A1) is the vdW energy, denoted as:
due to dipole–dipole (d–d) and dipole–quadruple (d–q) interactions. The abbreviations C and D represent the overall vdW coefficients, defined as36:
and
cij and dij are the vdW coefficients due to d–d and d–q interactions. We follow the variational method38 to derive cij and dij as:
and
Here, me is the electron mass, αi is the electronic polarizability, and Ni denotes the effective number of electrons of the ith ion. The values of the overall vdW coefficients are obtained using Eqs. (A5) and (A6), and weighted in terms of appropriate lattice sums [S6(0), S6(r), S8(0), and S8(r)].36 We believe that there is no uncertainty involved in the evaluation of cij and dij, due to the fact that the excitation energies are ignored in Eqs. (A6a) and (A6b). While applying for manganites herein, the above description, we shall seek the interionic interaction in between a pair such as Mn–O and La/A–O.
It is clear from the above descriptions that the present effective interionic potential contains only two free parameters (b and ρ), which are determined from the equilibrium conditions:
and Bulk modulus
While estimating these values, we use the Bulk modulus39 from experiment and r0 (obtained from Rietveld refinement of XRD data). The model parameters obtained from Eqs. (A7a) and (A7b) have been used to compute the SOECs (C11, C12, and C44) as40,41
where (A1, B1) and (A2, B2) are the short-range parameters for the nearest and the next nearest neighbors, respectively. These parameters are further defined as
where Vij(r) is the short-range potentials between the ions, which follow
The elastic force constant κ is derived at the equilibrium interionic distance r0 following
We have thus estimated the elastic force constants for a pair such as Mn–O and La/A–O and have the total force constants of the A-doped LaMnO3. This continuum model thus takes care of the clear physical binding in doped manganites incorporating vdW interactions. However, the true potential must recognize the correct charge distribution and the relative orientations of the interacting atoms in manganites which is a complicated task.
We shall now estimate the acoustic Debye branch characterized by the Debye temperature θD and an optical peak defined by the Einstein temperature θE. The Debye frequency is characterized as a cut off frequency at the Brillouin zone boundary. It can be expressed in terms of effective value of ionic mass and elastic force constant for crystal lattices with two different kinds of atoms such as Mn–O and La/A–O, which we deal with. The acoustic-mode and optical-mode frequencies are estimated in an ionic model using a value of effective ion charge Ze = −2e.
We choose an acoustic mass M = (2M+ + M−) [Mn (O) which is symbolized by M+(M−)], κ* = 2κ for each directional oscillation mode to get the acoustic phonon frequency as
Furthermore, when the phonons belong to optic modes, their frequency is determined by the reduced mass as μ−1 = M(A)−1 + M(B)−1 where A is the anion (La/A, Mn) and B is the cation (O)
where η is the force constant as
ωLO (ωTO) symbolized for the longitudinal (transverse) optical phonon frequency and Ω for the volume of the unit cell. We must mention that these computed values for La1−xAxMnO3 with 0.25 ≥ x ≥ 0.35 could not be compared due to the lack of the experimental data. However, the computed values of Debye and Einstein temperature for doped La0.7Ba0.3 MnO3 manganites are consistent with previous specific heat and Raman spectroscopic measurements.
It is true that the two-orbital model based on Wannier functions predicts the electronic states such as charge ordering in manganites. As mentioned, the Wannier function approach of the electronic problem is useful for the description of electron dynamics following a semiclassical theory as well as the magnetic interactions in solids.42 In the present investigations, we do not intend to discuss the electron dynamics as well the magnetic interactions, but focused on determining the acoustic (optical) phonon frequency to estimate the electron–phonon contribution of resistivity in the FMM phase.
Earlier, Millis43 determines the elastic parameters using a mean field approximation with emphasis on Mn–O bond lengths to evaluate the Mn–Mn and Mn–O force constants for the lattice distortions. However, we have considered both Mn–O and La/A–O bond lengths to obtain the Mn–O, La/A–O force constant, and total force constants for strong electron–phonon interaction. The formulated EIoIP includes the long-range Coulomb, vdW interaction, and the short-range repulsive interaction up to second-neighbor ions within the Hafemeister and Flygare approach. The interionic interaction in between a pair such as Mn–O and La/A–O enables us to find the total force constant with consistent Debye and Einstein temperatures.
The developed EIoIP thus takes care of the interactions in between a pair such as Mn–O and La/A–O. The interactions thus are attractive Coulomb, and vdW as well as short-range overlap repulsive interaction following Hafemeister and Flygare type potential. The advantage of using this potential is that it takes care of number of nearest (next nearest) ions, the valence, and number of the outermost electrons in the anions (cations), respectively. Thus, it takes care of the structural parameters that yield an approximately correct description of the interactions between a pair such as Mn–O and La/A–O. Henceforth, we are able to estimate the acoustic and optical phonon frequency consistent with the specific heat and Raman measurements to estimate the electron–phonon contribution of resistivity.
Rights and permissions
About this article
Cite this article
Varshney, D., Dodiya, N. Structural and magnetotransport studies of magnetic ion doping for monovalent-doped LaMnO3 manganites. Journal of Materials Research 29, 1183–1198 (2014). https://doi.org/10.1557/jmr.2014.103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2014.103