Abstract
Decoupled growth often occurs in the nonfacetted-facetted eutectic systems. And it is generally considered that the nonfacetted solid solution acts as the leading phase in the decoupled growth. In this work, Fe40Ni40P14B6 eutectic alloys were systematically studied via solidification of undercooled melts and crystallization of amorphous alloys. Upon solidification of melts subjected to different undercoolings, as the undercooling increases, the growth mechanism develops from cooperative growth to decoupled growth. Upon crystallization of amorphous alloys, the partially crystallized sample consists only of strongly faulted intermetallic (Fe,Ni)3(P,B) with chemical composition deviating from stoichiometry. Formation of supersaturated solid solution γ(Fe, Ni) in the solidification and supersaturated intermetallic (Fe,Ni)3(P,B) in the amorphous crystallization indicates that decoupled growth results from solute trapping and disorder trapping in rapid growth of solid solution and intermetallic, respectively. Further application of rapidly quenched experiments and theoretical analysis declare that the decoupled growth results from a competition between the growth of γ(Fe, Ni) and (Fe,Ni)3(P,B), which are controlled by solute trapping and disorder trapping, respectively.









Similar content being viewed by others
References
K.A. Jackson and J.D. Hunt: Lamellar and rod eutectic growth. Trans. Metall. Soc. AIME 236, 1129 (1966).
R. Trivedi, P. Magnin, and W. Kurz: Theory of eutectic growth under rapid solidification conditions. Acta Metall. 35, 971 (1987).
W. Kurz and R. Trivedi: Eutectic growth under rapid solidification conditions. Metall. Trans. A 22, 3051 (1991).
T.Z. Kattamis and M.C. Flemings: Structure of undercooled Ni-Sn eutectic. Metall. Mater. Trans. B 1, 1449 (1970).
B.L. Jones: Growth mechanisms in undercooled eutectics. Metall. Mater. Trans. B 2, 2950 (1971).
B. Wei, D.M. Herlach, B. Feuerbacher, and F. Sommer: Dendritic and eutectic solidification of undercooled Co-Sb alloys. Acta Metall. Mater. 41, 1801 (1993).
J.F. Li, X.L. Li, L. Liu, and S.Y. Lu: Mechanism of anomalous eutectic formation in the solidification of undercooled Ni-Sn eutectic alloy. J. Mater. Res. 23, 2139 (2008).
R. Goetzinger, M. Barth, and D.M. Herlach: Mechanism of formation of the anomalous eutectic structure in rapidly solidified Ni-Si, Co-Sb, and Ni-Al-Ti alloys. Acta Mater. 46, 1647 (1998).
J.F. Li, W.Q. Jie, S. Zhao, and Y.H. Zhou: Structural evidence for the transition from coupled to decoupled growth in the solidification of undercooled Ni-Sn eutectic melt. Metall. Mater. Trans. A 38, 1806 (2007).
P.K. Galenko and D.M. Herlach: Diffusionless crystal growth in rapidly solidifying eutectic systems. Phys. Rev. Lett. 96, 150602–150611 (2006).
S.C. Gill and W. Kurz: Rapidly solidified AlCu alloys-II. Calculation of the microstructure selection map. Acta Metall. Mater. 43, 139 (1995).
M.J. Aziz and W.J. Boettinger: On the transition from short-range diffusion-limited to collision-limited growth in alloy solidification. Acta Metall. Mater. 42, 527 (1994).
M.J. Aziz: Interface attachment kinetics in alloy solidification. Metall. Mater. Trans. A 27, 671 (1996).
M.J. Li and K. Kuribayashi: Nucleation-controlled microstructures and anomalous eutectic formation in undercooled Co-Sn and Ni-Si eutectic melts. Metall. Mater. Trans. A 34, 2999 (2003).
G.X. Wang and V. Prasad: Nonequilibrium phenomena in rapid solidification: Theoretical treatment for process modeling. Microscale Thermophys. Eng. 1, 143 (1997).
W.J. Boettinger and M.J. Aziz: Theory for the trapping of disorder and solute in intermetallic phases by rapid solidification. Acta Metall. 37, 3379 (1989).
W.L. Wang, Y.J. Lv, H.Y. Qin, and B. Wei: Growth rules of the dendrites in liquid Fe-Sb alloy. Sci. China, Ser. G 39, 357 (2009).
M.J. Aziz: Model for solute redistribution during rapid solidification. J. Appl. Phys. 53, 1158 (1982).
M.J. Aziz and T. Kaplan: Continuous growth model for interface motion during alloy solidification. Acta Metall. 36, 2335 (1988).
C.A. MacDonald, A.M. Malvezzi, and F. Spaepen: Picosecond time-resolved measurements of crystallization in noble metals. J. Appl. Phys. 65, 129 (1989).
W.J. Boettinger, L.A. Bendersky, J.A. West, M.J. Aziz, and J. Cline: Disorder trapping in Ni2TiAl. Mater. Sci. Eng., A 133, 592 (1991).
J.A. West: Kinetic disordering of intermetallic compounds through first and second order phase transitions by rapid solidification. Ph.D. Thesis, Harvard University, Cambridge, MA, 1993, p. 145.
Q. Li: Formation of bulk ferromagnetic nanostructured Fe40Ni40P14B6 alloys by metastable liquid spinodal decomposition. Sci. China, Ser. E Eng. Mater. Sci. 52, 1919 (2009).
C.Q. Zhang and K.F. Yao: Nanocrystalline structures of Fe-Ni-P-B alloy solidified at large undercooling and liquid spinodal decomposition. Acta Metall. Sinica 42, 870 (2006).
X.J. Han, N. Wang, and B. Wei: Rapid eutectic growth under containerless condition. Appl. Phys. Lett. 81, 778 (2002).
J.L. Walter, P. Rao, E.F. Koch and S.F. Bartram: A microstructural study of the crystallization of the amorphous alloy Ni40Fe40P14B6. Metall. Trans. A 8, 1141 (1977).
T. Watanabe and M.J. Scott: The crystallization of the amorphous alloy Fe40Ni40P14B6. J. Mater. Sci. 15, 1131 (1980).
J.F. Li and Y.H. Zhou: Eutectic growth in bulk undercooled melts. Acta Mater. 53, 2351 (2005).
S. Binder, M Kolbe, S. Klein, and D.M. Herlach: Solidification of tetragonal Ni2B from the undercooled melt. Eur. Phys. Lett. 97, 36003–36011 (2012).
V. Raghavan: B-Fe-Ni (boron-iron-nickel). J. Phase Equilib. Diffus. 28, 377 (2007).
L. Battezzati, C. Antonione, and M. Baricco: Undercooling of Ni-B and Fe-B alloys and their metastable phase diagrams. J. Alloys Compd. 247, 164 (1997).
K. Eckler, R.F. Cochrane, D.M. Herlach, and B. Feuerbacher: Non-equilibrium solidification in undercooled Ni-B alloys. Mater. Sci. Eng., A 133, 702 (1991).
P. Franke, D. Neuschütz, and Scientific Group Thermodata Europe (SGTE): The Landolt-Börnstein Database, Springer Materials. http://www.springermaterials.com, pp. 1–4
C.V. Thompson and F. Spaepen: Homogeneous crystal nucleation in binary metallic melts. Acta Metall. 31, 2021 (1983).
K. Zhang: Investigation on formation and further transformation of non-equilibrium structures for Fe-based alloys. Ph.D. Thesis, Northwestern Polytechnical University, China, 2013, p. 73.
T.D. Shen and R.B. Schwarz: Bulk ferromagnetic glasses in the Fe-Ni-P-B system. Acta Mater. 49, 837 (2001).
W.J. Boettinger, S.R. Coriell, and R. Trivedi: Application of dendritic growth theory to the interpretation of rapid solidification microstructures. In Rapid Solidification Processing: Principles and Technologies IV, R. Mehrabian and P.A. Parrish eds.; Claitor’s Publishing Division: Baton Rouge, LA, 1988; p. 13.
W. Kurz and D.J. Fisher: Fundamentals of Solidification (Trans Tech Publications Ltd, Aedermannsdorf, 1986), p. 325.
D. Gupta, K. Vieregge, and W. Gust: Interface diffusion in eutectic Pb-Sn solder. Acta Mater. 47, 5 (1999).
H.Q. Hu: Fundamentals of Metal Solidification (China Machine Press, Beijing, China, 1996), p. 247.
W. Dudzinski: J.P. Morniroli, and M.J. Gantois: Stacking faults in chromium, iron and vanadium mixed carbides of the type M7C3. J. Mater. Sci. 16, 1387 (1980).
Y.K. Wu, J.Z. Liang, and K.H. Kuo: Micro inversion domains formed during the crystallization of the amorphous alloy Fe40Ni40P14B6. Phys. Status Solidi A 64, 113 (1981).
R. Ahmad, R.F. Cochrane, and A.M. Mullis: Disorder trapping during the solidification of ßNi3Ge from its deeply undercooled melt. J. Mater. Sci. 47, 2411 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Address all correspondence to this author.
Appendix—Theoretical Analysis of Phase Competition
Appendix—Theoretical Analysis of Phase Competition
Crystalline growth of an alloy shows much more complexity than that of a pure material. Besides the advancement of the interface, one also needs to consider the solute redistribution across the interface.12 When the interface conditions deviate significantly from the local equilibrium, one needs to consider a nonequilibrium partition coefficient, k. Several models have been developed to correlate the nonequilibrium partition coefficient, k, with the interface conditions, and the most widely used model for a dilute binary alloy is of Aziz12:
where k is the ratio of the solute concentration in the growing solid (XS) to that in the liquid (XL) at the interface, ke is the equilibrium value of k, v is the interface velocity, and vd is the diffusive speed discussed above. From this equation, it is evident that when the interface velocity is much larger than the interface diffusion velocity, k approaches unity and "partitionless" solidification can occur.
For metallic alloys, a collision-limited relationship is assumed between velocity and driving force under all circumstances.
where ΔGeff is the entire free energy dissipated at the interface, R the universal gas constant, T the interfacial temperature and the kinetic prefactor, vs is the rate of the forward flux alone, and corresponds to the hypothetical maximum growth velocity at infinite driving force.
Since the undercooling is relatively small, solute diffusivity in undercooled free solidification is treated to be independent of underccoling for simplicity, and the values of vs and vd are assumed to be constants (in Aziz’s model,12vs is assumed to be the velocity of sound, much larger than vd). So the S-L interface velocity is only thermodynamic-controlled and increases continuously with the undercooling. However, this deviates a lot from Wang’s experiment19 about the solidification of Fe-10% Sb system, where the growth velocity of α-Fe dendrite does not always increase with the undercooling but for extremely large undercooling, decreases with the undercooling. So, for large undercooling (that is the case for amorphous crystallization), solute diffusivity is highly dependent on temperature, and vs and vd could not be treated as constants. Here, the Arrhenius relationship is assumed for vs and vd:
where vs0 and vd0 are constants and E is the activation energy.
If the liquid and solid phases are sufficiently dilute that Henry’s law is valid, an expression for ΔGeff can be obtained12 as
where \(X_{\text{S}}^{\text{e}}\) and \(X_{\text{L}}^{\text{e}}\) are the equilibrium values of XS and XL, respectively. If the phase diagram liquidus and solidus boundaries are linear for the compositions of interest, they can be described by
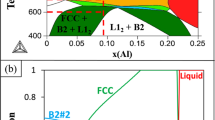
Eutectic system Fe40Ni40P14B6 consists of solid solution γ(Fe, Ni) and intermetallic (Fe,Ni)3(P,B). On one hand, because the solubility of Fe and Ni in intermetallic (Fe,Ni)3(P,B) is negligible compared to that of P and B in solid solution γ(Fe, Ni), the value of ke\(k_{\text{e}}^{{\text{II}}} >k_{\text{e}}^{\text{I}},X_{\text{L}}^{{\text{II}}} = X_{\text{L}}^{\text{I}}\) is smaller for intermetallic than for the solid solution. On the other hand, because the composition of eutectic point (Fe40Ni40P14B6) is much closer to that of the intermetallic (Fe,Ni)3(P,B) than γ(Fe, Ni), the value of XL is smaller for (Fe,Ni)3(P,B) than for γ(Fe, Ni), assuming that XL does not change during the transformation.
Free energy, ΔGeff has significant impact on interface velocity. Larger absolute value of ΔGeff/RT (ΔGeff/RT is negative) suggests larger interface velocity. Take the derivative of Eq. (A4) with respect to ke and XL, respectively.
It can be seen for Eq. (A6) that the increase in ke and the decrease in XL make the free energy and the interface velocity increase. Take the derivative of Eqs. (A6a) and (A6b) with respect to k, respectively.
It can be seen for Eq. (A7) that as k increases (induced by the increase in the growth velocity), the extent of influences of ke and XL on the growth velocity decreases and increases, respectively.
To further illustrate how ke and XL influence the growth velocity, the full numerical solution of the equation set [Eqs. (A1)-(A5)] is plotted in Fig. A1, which is interface temperature versus relative velocity (v/vs)or k for fixed XL. The parameters used in the calculation are listed in Table A1. Curve II and curve I correspond to the same XL value but to different ke values \(k_{\text{e}}^{{\text{III}}} = k_{\text{e}}^{\text{I}},X_{\text{L}}^{{\text{III}}} >X_{\text{L}}^{\text{I}}\), and the growth velocity of curve II is larger than that of curve I at the same temperature (Fig. A1a); curve I and curve III correspond to the same ke value but to different XL values \(k_{\text{e}}^{{\text{IV}}} >k_{\text{e}}^{\text{I}},X_{\text{L}}^{{\text{IV}}} >X_{\text{L}}^{\text{I}}\), and the growth velocity of curve I is larger than that of curve III at the same temperature (Fig. A1b); curve I and curve IV correspond to different ke and XL values \(\left( {X_{\text{S}}^{\text{e}}/X_{\text{L}}^{\text{e}}} \right)\), and the growth velocity of curve IV is larger than that of curve I when k is relatively small, the result is just opposite when k is relatively large (Fig. A1c). For Fe40Ni40P14B6 eutectic system, the intermetallic (Fe,Ni)3(P,B) has smaller values of ke and XL compared to the solid solution γ(Fe, Ni), which is like the case of curve I and curve IV (Fig. A1c). Therefore, as the undercooling increases (k increases), the leading phase in the decoupled growth of Fe40Ni40P14B6 eutectic system is γ(Fe, Ni) at first, and (Fe,Ni)3(P,B) at last. Moreover, as the leading phase changes from γ(Fe, Ni) to (Fe,Ni)3(P,B), the growth velocities of the two phases could probably become comparable with each other.
Interface temperature versus velocity, numerical solution of Eqs. (A1)-(A5) for fixed XL. To demonstrate how ke and XL influence the interface velocity, the different values of ke and XL are adopted: (a) \(\left( {k_{\text{e}}^{{\text{II}}} >k_{\text{e}}^{\text{I}}} \right)\); (b) \(\left( {X_{\text{L}}^{{\text{III}}} >X_{\text{L}}^{\text{I}}} \right)\); (c) \(\left( {k_{\text{e}}^{{\text{IV}}} >k_{\text{e}}^{\text{I}},X_{\text{L}}^{{\text{IV}}} >X_{\text{L}}^{\text{I}}} \right)\).
Rights and permissions
About this article
Cite this article
Gu, B., Liu, F., Dong, X. et al. Decoupled growth mechanism of Fe40Ni40P14B6 eutectic alloy. Journal of Materials Research 28, 2861–2873 (2013). https://doi.org/10.1557/jmr.2013.289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2013.289