Abstract
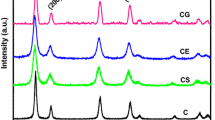
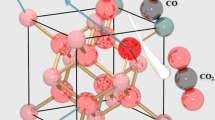
Effect of H2O2 on synthesis and powder properties such as surface area and agglomerate size of nanocrystalline Ce0.8M0.2O1.90 (M: Sm, Gd) was explored by treating cerium nitrate and rare-earth nitrate with NaOH in the presence/absence of H2O2. The resultant products were characterized by x-ray diffraction, Raman spectroscopy, thermo-gravimetry–differential thermal analysis, dynamic light scattering, surface area analysis, high-resolution transmission electron microscopy, and x-ray photoelectron spectroscopy. The presence of H2O2 was found to have a profound effect on powder properties such as surface area and particle size of these doped ceria samples and results in smaller crystallite size, softer agglomerates, and larger surface area. A mechanism is proposed to explain the observed better powder properties of the samples. It was also shown that the samples prepared in the presence of H2O2 can lower the conversion temperature of CO to CO2, proving these to be better catalysts. Interestingly, temperature-programmed reduction studies on Sm3+-doped samples showed that the doping in conjunction with the use of H2O2 leads to enhanced reduction properties of the samples over multiple cycles.
Similar content being viewed by others
References
S.J. Park, J.M. Vohs and R.J. Gorte: Direct oxidation of hydro-carbons in a solid-oxide fuel cell. Nature 265, 404 (2000).
E.P. Murray, T. Tsai and A. Barnett: A direct-methane fuel cell with a ceria-based anode. Nature 400, 649 (1999).
Y. Maki, M. Matsuda and T. Kudo: U.S. Patent No. 3 [607] 424, (1971).
V.V. Kharton, F.M. Figueiredo, L. Navarro, E.N. Naumovich, A.V. Kovalevsky, A.A. Yaremchenko, A.P. Viskup, A. Carneiro, F.M.B. Marques and J.R. Frade: Ceria-based materials for solid oxide fuel cells. J. Mater. Sci. 36, 1105 (2001).
A.I.Y. Tok, L.H. Luo, F.Y.C. Boey and S.H. Ng: Consolidation and properties of Gd0.1Ce0.9O1.95 nanoparticles for solid oxide fuel cell electrolytes. J. Mater. Res. 21, 19 (2006).
N. Izu, W. Shin and N. Murayama: Fast response of resistive-type oxygen gas sensors based on nano-sized ceria powder. Sens. Actuators, B 93, 449 (2003).
N.B. Kirk and J.V. Wood: Glass polishing. Br. Ceram. Trans. 93, 25 (1994).
X. Yin, L. Hong and Z-L. Liu: Development of oxygen transport membrane La0.2Sr0.8CoO3d/Ce0.8Gd0.2O2d on the tubular CeO2support. Appl. Catal., A 300, 75 (2006).
X. Yin, L. Hong and Z-L. Liu: Oxygen permeation through the LSCO-80/CeO2 asymmetric tubular membrane reactor. J. Membr. Sci. 268, 2 (2006)
S. Damayanova and J.M.C. Bueno: Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts. Appl. Catal., A 253, 135 (2003).
Y.F. Yao and J.T. Kummer: Low-concentration supported precious metal catalysts prepared by thermal transport. J. Catal. 106, 307 (1987).
E.C. Su, C.N. Montreuil and W.G. Rothschild: Oxygen storage capacity of monolith three-way catalysts. Appl. Catal. 17, 75 (1985).
S. Imamura, I. Fukuda and S. Ishida: Wet oxidation catalyzed by ruthenium supported on cerium (IV) oxides. Ind. Eng. Chem. Res. 27, 718 (1988).
V.S. Mishra, V.V. Mahajani and J.B. Joshi: Wet air oxidation. Ind. Eng. Chem. Res. 34, 2 (1995).
A. Trovarelli, C. de Leittenburg, M. Boaro and G. Dolcetti: Redox chemistry over CeO2-based catalysts: SO2 reduction by CO or CH4. Catal. Today 50, 381 (1999).
S. Zhao and R.K. Gorte: A comparison of ceria and Sm-doped ceria for hydrocarbon oxidation reactions. Appl. Catal., A 277, 129 (2004).
S. Kawi, Y.P. Tang, K. Hidajat and L.E. Yu: Synthesis and characterization of nanoscale CeO2 catalyst for deNOx. J. Meta-stable Nanocryst. Mater. 23, 95 (2005).
M.K. Neylon, M.J. Castagonla, N.B. Castagonla and C.L. Marshall: Coated bifunctional catalysts for NOx SCR with C3H6: Part I: Water-enhanced activity. Catal. Today 96, 53 (2004).
G. Colon, J.A. Navio, R. Monaci and I. Ferino: CeO2–La2O3catalytic system Part I. Preparation and characterisation of catalysts. Phys. Chem. Chem. Phys. 2, 4453 (2000).
K. Krishna, A. Bueno-Lopez, M. Makkee and J.A. Moulijn: Potential rare earth modified CeO2 catalysts for soot oxidation. I. Characterisation and catalytic activity with O2. Appl. Catal. B 75, 189 (2007).
U. Hennings and R. Reimert: Noble metal catalysts supported on gadolinium doped ceria used for natural gas reforming in fuel-cell applications. Appl. Catal. B 70, 498 (2007).
F.X. Liu, C.Y. Wang, Q.D. Su, T.P. Zhao and G.W. Zhao: Optical properties of nanocrystalline ceria. Appl. Opt. 36, 2796 (1997).
Y.M. Chiang, E.B. Lavik and D.A. Blom: Defect thermodynamics and electrical properties of nanocrystalline oxides: Pure and doped CeO2. Nanostruct. Mater. 9, 633 (1997).
Z. Yanchun and M.N. Rahaman: Effect of redox reaction on the sintering behavior of cerium oxide. Acta Mater. 45, 3635 (1997).
A. Tschope, D. Schaadt, R. Birringer and J.Y. Ying: Catalytic properties of nanostructured metal oxides synthesized by inert gas condensation. Nanostruct. Mater. 9, 423 (1997).
C. Laberty-Robert, J.W. Long, E.M. Lucas, K.A. Pettigrew, R.M. Stroud, M.S. Doescher and D.R. Rolison: Sol-gel-derived ceria nanoarchitectures: Synthesis, characterization, and electrical properties. Chem. Mater. 18, 50 (2006).
F. Deganello, V. Esposito, M. Miyayama and E. Traversa: Cathode performance of nanostructured La1-aSraCo1-bFebO3-x on a Ce0.8Sm0.2O2 electrolyte prepared by citrate-nitrate autocombustion. J. Electrochem. Soc. 154, A89 (2007).
D.E. Wesolowski and M.J. Cima: Nitrate-based metalorganic decomposition of CeO2 on yttria-stabilised zirconia. J. Mater. Res. 21, 1 (2006).
F. Deganello, L.F. Lotta, A. Longo, M.P. Casaletto and M. Scopelitti: Cerium effect on the phase structure, phase stability and redox properties of Ce-doped strontium ferrates. J. Solid State Chem. 179, 3406 (2006).
J.C. Yu, L.Z. Zhang and J. Lin: Direct sonochemical preparation of high surface area nanoporous ceria and ceria–zirconia solid solutions. J. Colloid Interface Sci. 260, 240 (2003).
T. Tsuzuki and P.G. McCormick: Synthesis of ultrafine ceria powders by mechanochemical processing. J. Am. Ceram. Soc. 84, 1453 (2001).
H.R. Xu, L. Gao, H.C. Gu, J.K. Guo and D.S. Yan: Synthesis of solid, spherical CeO2 particles prepared by the spray hydrolysis reaction method. J. Am. Ceram. Soc. 85, 39 (2002).
P.L. Chen and I.W. Chen: Reactive cerium(IV). Oxide powders by the homogeneous precipitation method. J. Am. Ceram. Soc. 76, 1577 (1993).
H.I. Chen and H.Y. Chang: Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids Surf., A 242, 61 (2004).
M. Ozawa: Effect of aging temperature on CeO2 formation in homogeneous precipitation. J. Mater. Sci. 39, 4035 (2004).
J.L. Woodhead: Process for preparing aqueous dispersion of ceria and resulting product. U.S. Patent No. 4231893, November 4, 1980.
B. Djuricic and S. Pickering: Nanostructured cerium oxide: Preparation and properties of weakly-agglomerated powders. J. Eur. Ceram. Soc. 19, 1925 (1999).
J.S. Lee and S.C. Choi: Crystallization behavior of nano-ceria powders by hydrothermal synthesis using a mixture of H2O2 and NH4OH. Mater. Lett. 58, 390 (2004).
B.P. Mandal, V. Grover and A.K. Tyagi: Phase relations, lattice thermal expansion in Ce1-xEuxO2-x/2 and Ce1-xSmxO2-x/2 systems and stabilization of cubic RE2O3 (RE: Eu, Sm). Mater. Sci. Eng., A 430, 120 (2006).
V. Grover and A.K. Tyagi: Phase relations, lattice thermal expansion in CeO2-Gd2O3 system, and stabilization of cubic gadolinia. Mater. Res. Bull. 39, 859 (2004).
F.H. Scholes, A.E. Hughes, S.G. Hardin, P. Lynch and P.R. Miller: Influence of hydrogen peroxide in the preparation of nanocrystalline ceria. Chem. Mater. 19, 2321 (2007).
T. Moeller: The Chemistry of Lanthanides (Pergamon Press, Oxford, 1973).
B.P. Mandal, V. Grover, M. Roy and A.K. Tyagi: X-ray diffraction and Raman spectroscopic investigation on the phase relations in Yb2O3- and Tm2O 3-substituted CeO2. J. Am. Ceram. Soc. 90, 2961 (2007).
B.P. Mandal, M. Roy, V. Grover and A.K. Tyagi: X-ray diffraction, m-Raman spectroscopic studies on CeO2-RE2O3 (RE=Ho, Er) systems: Observation of parasitic phases. J. Appl. Phys. 103, 033506 (2008).
A. Nakajima, A. Yoshihara and M. Ishigame: Defect-induced Raman spectra in doped CeO2. Phys. Rev. B 50, 13297 (1994).
W.H. Weber, K.C. Hass and J.R. McBride: Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 48, 178 (1993).
F. Zhang, W.S. Chan, J.E. Spanier, E. Apak, Q. Jin, R.D. Robinson and I.P. Herman: Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 80, 127 (2002).
Q. Williams: Mineral Physics and Crystallography: A Handbook of Physical Constants (American Geophysical Union, Washington, DC, 1995).
J. Guzman, S. Carrettin and A. Corma: Spectroscopic evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nanocrystalline CeO2. J. Am. Chem. Soc. 17, 3286 (2005).
V.V. Pushkarev, V.I. Kovalchuk and J.L. d’Itri: Probing defect sites on the CeO2 surface with dioxygen. J. Phys. Chem. B 108, 5341 (2004).
A.J. Simaan, S. Dopner, F. Banse, S. Bourcier, G. Bouchoux, A. Boussac, P. Hildebrandt and J. Girerd: FeIII-hydroperoxo and peroxo complexes with aminopyridyl ligands and the resonance raman spectroscopic identification of the Fe-O and O-O stretching modes. Eur. J. Inorg. Chem. 1627 (2000).
P.A. Giguere and T.K.K. Srinivasan: Raman study of matrix isolated H2O2 and D2O2. Chem. Phys. Lett. 33, 479 (1975).
S. Banerjee, P.S. Devi, D. Topwal, S. Mandal and K. Menon: Enhanced ionic conductivity in Ce0.8Sm0.2O1.9: Unique effect of calcium Co-doping. Adv. Funct. Mater. 17, 2847 (2007).
A.E. Hughes, R.J. Taylor, B.R.W. Hinton and L. Wilson: XPS and SEM characterization of hydrated cerium oxide conversion coatings. Surf. Interface Anal. 23, 540 (1995).
F. Giordano, A. Trovarelli, C. de Leitenburg and M. Giona: A model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 193, 273 (2000).
H. He, H. Dai and C.T. Au: Defective structure, oxygen mobility, oxygen storage capacity, and redox properties of RE-based (RE = Ce, Pr) solid solutions. Catal. Today 90, 245 (2004).
M. Pijolat, M. Prin and M. Soustelle: Thermal stability of doped ceria: Experiment and modeling. J. Chem. Soc. Faraday Trans. 91, 3941 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, B.P., Grover, V., Pai, M.R. et al. Improvement of physico-chemical properties by addition of H2O2: An extensive case study on the RE-doped ceria system (RE = Gd, Sm. Journal of Materials Research 24, 2845–2854 (2009). https://doi.org/10.1557/jmr.2009.0352
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2009.0352