Abstract
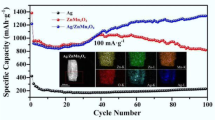
Ag7Fe3(P2O7)4 is a 3D structured material which has been recently studied as a possible cathode material for lithium batteries. Notably, Na7Fe3(P2O7)4 is reported to be a fast-ion conductor, yet poor electrical conductor. Here, partial replacement of Na+ for Ag+ yielded Na2Ag5Fe3(P2O7)4 pyrophosphate framework where the formation of Ag metal is proposed to increase the intrinsic low electrical conductivity of this polyanion electrode. Specifically, the Ag5Na2Fe3(P2O7)4 -Ag composite is synthesized via chemical reduction of Ag7Fe3(P2O7)4 using NaBH4. The occupancy of Ag+ and Na+ in each site was determined via Rietveld analysis of the diffraction pattern. Electrochemistry of the Ag5Na2Fe3(P2O7)4 -Ag metal composite was explored with voltammetry and galvanostatic charge/discharge cycling. The Ag5Na2Fe3(P2O7)4 -Ag metal composite electrodes displayed good rate capability assisted by the presence of Ag metal from the chemical reduction and in-situ electrochemical formation of a Ag conductive network.
Similar content being viewed by others
References
M. Armand and J. M. Tarascon, Nature 451 (7179), 652–657 (2008).
M. Tamaru, S. C. Chung, D. Shimizu, S.-I. Nishimura and A. Yamada, Chem. Mater. 2013 (25), 2538–2543 (2013).
A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc. 144 (4), 1188–1194 (1997).
S. Nishimura, M. Nakamura, R. Natsui and A. Yamada, Journal of the American Chemical Society 2010 (132), 13596–13597 (2010).
D. Shimizu, S.-i. Nishimura, P. Barpanda and A. Yamada, Chem. Mater. 24 (13), 2598–2603 (2012).
H. Kim, S. Lee, Y.-U. Park, H. Kim, J. Kim, S. Jeon and K. Kang, Chemi. Mater. 2011 (23), 3930–3937 (2011).
N. Furuta, S.-i. Nishimura, P. Barpanda and A. Yamada, Chem. Mater. 24 (6), 1055–1061 (2012).
A. K. Padhi, K. S. Nanjundaswamy, C. Masquelier, S. Okada and J. B. Goodenough, J. Electrochem. Soc. 144 (5), 1609–1613 (1997).
C. Wurm, M. Morcrette, G. Rousse, L. Dupont and C. Masquelier, Chem. Mater. 14 (6), 2701–2710 (2002).
J. Cabana, J. Shirakawa, M. Nakayama, M. Wakihara and C. P. Grey, J. Mater. Chem. A 21 (27), 10012–10020 (2011).
H. Gao, S. Zhang and C. Deng, Dalton Trans. 44 (1), 138–145 (2015).
S. Y. Chung, J. T. Bloking and Y. M. Chiang, Nature Mater. 2002 (1) (2002).
I. Boyano, J. A. Blazquez, I. de Meatza, M. Bengoechea, O. Miguel, H. Grande, Y. H. Huang and J. B. Goodenough, J. Power Sources 195 (16), 5351–5359 (2010).
Z. L. Liu, S. W. Tay, L. A. Hong and J. Y. Lee, Journal of Solid State Electrochemistry 15 (1), 205–209 (2011).
D. Gryzlov, S. Novikova, T. Kulova, A. Skundin and A. Yaroslavtsev, Mater. Design 104, 95–101 (2016).
H. Goktepe, H. Sahan and S. Patat, Inter. J. Hydrogen Energy 41 (23), 9774–9779 (2016).
E. S. Takeuchi, A. C. Marschilok, K. Tanzil, E. S. Kozarsky, S. Zhu and K. J. Takeuchi, Chem. Mater. 2009 (21) (20), 4934–4939 (2009).
K. C. Kirshenbaum, D. C. Bock, A. B. Brady, A. C. Marschilok, K. J. Takeuchi and E. S. Takeuchi, Phys. Chem. Chem. Phys. 17 (17), 11204–11210 (2015).
Y. Zhang, K. C. Kirshenbaum, A. C. Marschilok, E. S. Takeuchi and K. J. Takeuchi, Chem. Mater. 2016 (28), 7619–7628 (2016).
E. Quarez, O. Mentre, K. Djellab and C. Masquelier, New J. Chem. 34 (2), 287–293 (2010).
C. Masquelier, F. Dyvoire and N. Rodier, J. Solid State Chem. 95 (1), 156–167 (1991).
E. Quarez, O. Mentre, Y. Oumellal and C. Masquelier, New J. Chem. 33 (5), 998–1005 (2009).
B. H. Toby, J. Appl. Crystallogr. 34, 210–213 (2001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Marschilok, A.C., Takeuchi, E.S. et al. Preparation and structure of Na2Ag5Fe3(P2O7)4 -Ag metal composite: Insights on electrochemistry. MRS Advances 2, 395–400 (2017). https://doi.org/10.1557/adv.2017.56
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2017.56