Abstract
α-LiFeO2 is a promising cathode material for lithium-ion batteries due to its theoretically high specific capacity (282 mAh g−1), abundant nature, low cost of raw materials and environmental friendliness. However, the intrinsic sluggish kinetics and poor electronic conductivity of α-LiFeO2 prevent its practical use. In this work, we introduce a novel electrostatic layer-by-layer self-assembly method using PAH and PSS charged polyelectrolytes to grow in situ Ag nanoparticles on the surface of α-LiFeO2 nanorods to improve the electronic and ionic conductivity in this material. The experimental results show that such tailored design effectively improves the cycling stability and provides the material with a superior rate capability. The Ag-1D α-LiFeO2 material delivers a high discharge capacity of 162.6 mAh g−1 at 0.5 C and a capacity retention of 89.6% after 50 cycles. The excellent electrochemical behavior may be ascribed to synergistic effects which combine the use of Ag NPs, which provide with improved electronic conductivities, and the large specific surface areas given by the 1D morphology of the nanorods, providing increased lithium and electron conduction pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rechargeable lithium-ion batteries (LIBs) have become the predominant power device for portable electronic products, electric and hybrid vehicles and smart grid applications [1, 2]. However, with rapid technological developments and serious environmental concerns, it is necessary to explore new cathode materials with high energy density, long cycle life, low toxicity and low production costs [2]. The commercialized LiCoO2 cathode material provides stable cycling and rate performance, but the unethically sourced cobalt coupled with its toxic nature poses a problem for its future use [3]. Thus, alternative cathode materials are ought to be explored. α-LiFeO2, with a disordered rock-salt structure, has been considered as a potential candidate to replace LiCoO2 due to its high theoretical capacity (282 mAh g−1), high elemental abundance, the low cost of the raw materials, environmental friendliness and safety during operation [4,5,6,7,8,9]. Unfortunately, α-LiFeO2 suffers from an intrinsically poor electronic conductivity and slow lithium-ion diffusion kinetics. To this end, various strategies have been developed to improve the conductivity and lithium-ion diffusivity of α-LiFeO2. Generally, nanostructured electrodes with one-dimensional (1D) morphology (e.g., nanorods, nanotubes and nanowires) have improved lithium storage performances due to their architecture, which facilitates electron transportation [10,11,12,13,14]. Furthermore, it has been demonstrated that modification with conductive additives can facilitate electron transport between the particles. Typically, researchers have used carbonaceous materials such as carbon from different organic sources and MWCNTs as conductive additives for α-LiFeO2 [15,16,17,18]. A different approach has been to use metals such as silver and copper to decorate the surface of the electrode material to improve the electronic conductivity of the composite material [19,20,21]. Silver has been used as a conductive additive in cathode materials such as LiMn2O4/Ag, LiFePO4/Ag, Co3O4/Ag and Si/Ag composites [22,23,24,25,26,27] given its high electrical conductivity (6.30 × 107 S m−1). However, it is difficult to precisely control the uniformity and particle size of the additives when using traditional methods such as liquid precipitation, in situ synthesis and silver mirror reactions [28,29,30]. By contrast, layer-by-layer self-assembly (LBL) technology, which is a technique that lies on the deposition of alternating layers of oppositely charged materials is an efficient method that may provide with a uniform particle distribution [31, 32].
In this work, we demonstrate a novel, low-cost, simple and scalable method to prepare 1D α-LiFeO2 modified with Ag NPs based on an electrostatic layer-by-layer self-assembly process using PAH and PSS polyelectrolytes. The results from structural and electrochemical analyses show that the Ag NPs were grown in situ on the surface of the α-LiFeO2 nanorods. As a result, enhanced ionic and electronic conductivities which enabled improved electrochemical performance in terms of cycling capability and rate performance were observed. This design strategy opens the pathway to the fabrication of new electrode designs with improved electrochemical properties.
Experiment section
Synthesis
Synthesis of α-LiFeO2 nanorods
α-LiFeO2 nanorods were prepared through a self-sacrificial template route using α-FeOOH nanorods [33]. The detailed experimental process for the synthesis of α-FeOOH nanorods is shown in the SI (Methods), and PXRD data can be found in Fig. S1. Then, 0.5 g α-FeOOH, 0.47 g LiOH⋅H2O and 0.78 g LiNO3 (Li/Fe = 4:1 molar ratio) were ground together with a mortar and pestle using a small amount of distilled water and anhydrous alcohol. After this, powders were dried at 80 °C for 12 h and then sintered at 400 °C for 6 h in a N2 atmosphere using a heating rate of 3 °C min−1. After naturally cooling down to room temperature, the product was washed with distilled water and ethanol and dried at 80 °C for 12 h. For comparison, α-LiFeO2 particles were prepared by a traditional solid-state method using LiOH⋅H2O and Fe2O3 (Li/Fe = 1.05:1 molar ratio) as precursors. The precursors were sintered at 800 °C for 8 h in a muffle furnace with a ramp of 3 °C/min and automatically cooled down to room temperature to obtain α-LiFeO2. PXRD and SEM data can be found in Fig. S2 and Fig. S3, respectively.
Preparation of Ag-1D α-LiFeO2 nanocomposite by electrostatic self-assembly
Typically, 0.25 g of as-synthesized 1D α-LiFeO2 were stirred for 1 h in 120 ml of a 0.5 M NaCl solution and after that 0.3 g of PSS (sodium-4-styrene sulfonate) were added and stirred for another 0.5 h. Then, the sample was washed repeatedly with deionized water to remove the excess of PSS. The same procedure was carried out using 0.3 g of PAH (poly(allylamine hydrochloride)), and this was repeated with PSS. As a result, PSS/PAH/PSS-modified 1D α-LiFeO2 was prepared. The modified 1D α-LiFeO2 nanorods were placed into 50 mL water with 0.005 g AgNO3 and 2.0 g polyvinyl pyrrolidone (PVP) and were stirred for 1 h. After this, 20 mL of a 0.025 M NaBH4 solution were added dropwise into the layer-by-layer modified sample. After stirred for 1 h, the resulting products were washed with distilled water and ethanol repeatedly and dried at 80 °C for 12 h. Figure 1 depicts a schematic of the electrostatic self-assembly process for the preparation of Ag-1D α-LiFeO2.
Characterization
Structural characterization
Powder X-ray diffraction (PXRD) was performed using a Bruker DX-1000, equipped with a Cu Kα radiation source (λ1 = 1.54056 Å, λ2 = 1.54439 Å) and a 2D detector.
Microstructural characterization
SEM (JEOL, JSM-6360LV) was performed using an accelerating voltage of 60 kV to determine the microstructure of the as-prepared α-LiFeO2 samples. TEM images were obtained using a JEOL, JEM-3010 at an accelerating voltage of 200 kV. TEM mapping images were obtained on an FEI Talos F200X with a field emission gun operating at 200 kV.
Zeta potential measurements
The zeta potentials of the Ag-1D α-LiFeO2 suspension liquids were determined using a Zetasizer Nano ZS90 equipment.
X-ray Photoemission Spectroscopy (XPS)
X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI) was measured on an Ultra DLD using a monochromic Al X-ray source. The electronic conductivity is measured by a digital four-point probe tester (SX1934 (SZ-82)).
Electrochemical studies
The electrochemical performance of the 1D α-LiFeO2 samples and pristine material was tested by using CR2025 coin-type cells, which were assembled in an Ar-filled glove box. Electrode slurries were made by mixing 85 wt% active material, 10% acetylene black (conductive additive) and 5% polyvinylidene difluoride (PVDF) (binder) with a mortar and pestle. The mixture was added into an N-methyl pyrrolidone solution that was stirred for 12 h. Then, the slurry was evenly coated onto aluminium foil with the help of a Doctor blade, and the films were dried at 80 °C for 30 min, followed by punching them into electrodes with a diameter of 12 mm. The electrodes were further dried at 100 °C for 12 h under vacuum before assembling them into coin cells. A loading of ca. 1.3 mg cm−2 was achieved in the electrodes of this study. For coin cell assembly, lithium foil was used as the counter and reference electrode, a Celgard microporous polypropylene membrane was used as a separator, and 1 M lithium hexafluorophosphate (LiPF6) dissolved in ethylene carbonate (EC) and diethyl carbonate (DEC) in a 1:1 volume ratio was used as the electrolyte. Galvanostatic measurements were performed on a Land CT2001A battery testing system at different current densities between 1.5 and 4.8 V (vs. Li+/Li). Electrochemical impedance spectroscopy (EIS) analysis was conducted on a CS-350 electrochemical workstation with a frequency range of 0.1 Hz to 100 kHz at an amplitude of 5 mV. Cyclic voltammetry (CV) was conducted at a scan rate of 0.1 mV s−1 in the 1.5–4.8 V voltage range.
Results and discussion
Figure 1 describes the electrostatic self-assembly approach for the synthesis of Ag-1D α-LiFeO2, where poly(allylamine hydrochloride) (PAH) and poly(sodium 4-styrenesulfonate) (PSS) were used as positively and negatively charged polyelectrolytes, respectively. The polyelectrolytes were used to homogeneously coat the 1D α-LiFeO2 based on a self-assembly process. The PAH-PSS couple is characterized by a strong hydrophobic electrostatic interaction between the ammonium group \(\left[ { - {\text{NH}}_{3}^{+} } \right]\) of PAH and the sulfonate group \(\left[ { - {\text{SO}}_{3}^{2 - } } \right]\) of PSS, leading to a high charge density [31, 32]. A similar approach using poly(diallyl dimethylammonium chloride) (PDDA) and poly(sodium-4-styrene sulfonate) (PSS) polyelectrolyte couples was used to coat a 3D carbon substrate with LiFePO4 nanoparticles [34].
During the PSS/PAH/PSS dipping cycles, we observed changes in the surface charge of the 1D α-LiFeO2 nanorods, going from negative (zeta potential = − 7.91 mV) to positive (zeta potential = 9.0 mV) and then back to negative (zeta potential = − 16.32 mV) (Fig. 2a), indicating the formation of opposite charges on the 1D α-LiFeO2 sample. At this point, positively charged Ag+ ions (from a 5.8 × 10−4 M AgNO3 solution) were adsorbed onto the surface of 1D α-LiFeO2 nanorods due to the electrostatic attraction between these and PSS. A solution of NaBH4 was then used to reduce in situ the Ag+ ions to Ag NPs onto the surface of 1D α-LiFeO2 nanorods.
a Zeta potentials of 1D α-LiFeO2 nanorods coated with PSS/PAH/PSS; b crystal structure of α-LiFeO2, showing a disordered distribution of Li/Fe atoms in octahedral sites in a cubic-close packing of oxygen atoms; c XRD data of 1D α-LiFeO2; and d XRD data of Ag-1D α-LiFeO2. hkl indexes are shown in c and d
PXRD data of the 1D α-LiFeO2 and Ag-1D α-LiFeO2 samples are shown in Fig. 2c and d. Both samples show diffraction peaks that can be indexed to the layered structure of α-LiFeO2 (Space group \(Fm\overline{3}m\), JCPDS No. 74–2284) and with lattice parameters in agreement with those reported in the literature [35]. The Ag-1D α-LiFeO2 sample shows an additional peak at 38.4° 2θ (marked in red), which matches to the most intense reflection of Ag (JCPDS No. 65–2871) [36] and thus confirms the presence of silver nanoparticles in the sample. No diffraction peaks from the α-FeOOH precursor were observed, indicating that these nanorods are fully converted into α-LiFeO2 Furthermore, PXRD data confirm that the LBL process does not change the crystal structure of the α-LiFeO2 nanorods, and thus, no side reactions occurred. Figure 2b shows the crystal structure of α-LiFeO2.
Micrographs of the samples are shown in Fig. 3a–d. Figure 3a shows a typical SEM image of the as-synthesized α-FeOOH sample, which consists of nanorods with diameters up to ca. 150 nm and lengths of a few micrometers. The micrographs indicate that the morphology of the α-LiFeO2 nanorods is retained after the thermal treatment on the α-FeOOH nanorods (Fig. 3b). Similarly, SEM images revealed that there are no changes in the diameter and morphology of the α-LiFeO2 nanorods after the LBL process (Fig. 3c, d) and showed the presence of Ag nanoparticles of a few nanometers uniformly coated on the surface of the 1D-α-LiFeO2.
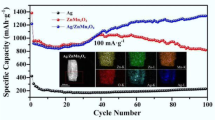
TEM analysis further confirms the detailed microstructure of 1D α-LiFeO2 and the distribution of the crystalline Ag nanoparticles onto the surface of 1D α-LiFeO2. Figure 4a shows a distinct α-LiFeO2 nanorod with a diameter of ca. 150 nm, which is fully consistent with the SEM data (Fig. 3b). The HRTEM image in Fig. 4b shows well-resolved lattice fringes, illustrating the high crystalline nature of the 1D α-LiFeO2 nanorods. The lattice fringe spacing of 0.24 nm is consistent with the (111) crystal planes of α-LiFeO2 [15]. Figure 4c and d shows high-magnification images that reveal round NPs with diameters of ca. 5 nm attached to the surface of 1D α-LiFeO2. The (111) crystal plane with an interplanar distance of 0.255 nm marked in Fig. 4e indicates the presence of silver nanoparticle [37]. The corresponding SAED pattern presented in Fig. 4f shows the diffraction rings formed from scattered dots, indexed from inside to outside as (111), (200), (220), (311), which are in good agreement with the XRD results. Based on TEM evidence, it can be found the Ag NPs are in the nano-range and uniformly distributed on the surface of the 1D α-LiFeO2 nanorods, suggesting a desirable architecture beneficial for fast electron transportation along the nanorods [30]. TEM-EDS mapping analysis shows a uniformly elemental distribution of O, Fe and Ag in the 1D-α-LiFeO2 and Ag-1D α-LiFeO2 nanorods (Fig. 5). Furthermore, the uniform coating of Ag nanoparticles is further confirmed in Ag-1D α-LiFeO2, suggesting that Ag nanoparticles can be evenly coated onto 1D α-LiFeO2 through a simple LBL approach.
X-ray photoelectron spectra (XPS) of the 1D α-LiFeO2 and Ag-1D α-LiFeO2 samples are shown in Fig. 6. According to the spectrum in Fig. 6a, Ag-1D α-LiFeO2 consists of Li, O, Fe and Ag peaks, which is in agreement with the formation and deposition of Ag NPs on the surface of 1D α-LiFeO2. Figure 6b shows Fe 2p peaks for both samples locating at 711.04 and 724.56 eV, which are assigned to Fe 2p3/2 and Fe 2p1/2 spin–orbit peaks of α-LiFeO2, respectively, thus confirming the Fe(III) oxidation state [38]. The two main peaks are accompanied by satellite peaks on their respective higher binding energy sides at about 719.8 and 733.4 eV [38]. No peak shift occurs to α-LiFeO2 before and after the LBL process, indicating that the LBL process does not change the chemical state of Fe. The high-resolution XPS spectra of the Ag doublet (3d3/2 and 3d5/2) in the Ag-1D α-LiFeO2 nanocomposites are shown in Fig. 6c. The doublet is centered at 368.3 and 374.2 eV, which are higher than those of metallic Ag (0) (3d3/2 = 367.9 eV; 3d5/2 = 373.9 eV) [39], suggesting a successful reduction of Ag(I) ions to Ag (0) through the LBL process.
The electrochemical performance of the Ag-1D α-LiFeO2 and 1D α-LiFeO2 materials as positive for LIBs was evaluated in coin cells using lithium as the counter and reference electrode. Typical charge/discharge profiles in the voltage range 1.5–4.8 V at 0.5 C current rate are shown in Fig. 7a and b. The 1st cycle load profiles are similar on both samples. The voltage increases rapidly from the open-circuit voltage to 4.5 V, followed by a plateau (40 mAh g−1) before reaching the cutoff voltage.
Load curves using a 0.5 C rate in the voltage range (1.5–4.8 V) of Ag-1D α-LiFeO2 (a) and 1D α-LiFeO2 (b); specific capacity versus cycle number plots in the voltage range of 1.5–4.8 V at 0.5 C (c); and 0.1 C (d). Coulombic efficiencies are shown in c and d; (e) Rate performance of the α-LiFeO2 samples at 0.1 C, 0.2 C, 0.5 C, 1 C and 2 C (e); and f average specific capacity at various current densities
During the first discharge, the voltage decreases sharply to 2.75 V and then enters a semi-plateau region that extends to 1.75 V, followed by a plateau in the 1.75–1.5 V region. Subsequent charge/discharge curves were S-shaped, as observed in other α-LiFeO2 materials [40]. Cyclic voltammetry was carried out to better understand the cycling behavior and redox processes occurring in the Ag-1D α-LiFeO2 material using a sweep rate of 0.1 mV s−1 between 1.5 and 4.8 V (vs. Li+/Li) (Fig. S4). We observed an anodic peak at 4.3 V during the first charge process which is consistent with the literature and the load curves (Fig. 7a) [41]. This peak disappears in the following cycles. Furthermore, the cathodic peak at 1.55 V decreased in intensity from the first to the second cycle. All the peaks maintained the same shape and current intensity after the first cycle. Based on this result, we assume that α-LiFeO2 underwent a structural change during the first cycle and maintained the changed structure after the second cycle. Several works from other researchers [4, 5, 16, 40, 42, 43] have explained the mechanism of structural evolution during the first and consequent cycles. Sakurai et al. [4, 42] suggested that unstable Fe4+ forms during the first charging process and oxidizes the electrolyte to produce Fe3+ ions. It should be noted that not all the Fe3+ oxidizes to Fe4+ and thus the low capacity observed during the first cycle. Upon discharge and consequent cycles, the predominant redox reaction is that coming from the relatively more stable Fe3+/Fe2+ redox couple. Furthermore, Morales et al. [43] have proposed a structural rearrangement upon lithium removal, where Fe4+ were displaced from octahedral 4a sites to tetrahedral 8c positions. The 1D α-LiFeO2 sample exhibits a first cycle discharge capacity of 151.2 mAh g−1 and retains 73.7% of this capacity after 50 cycles. By contrast, a discharge capacity of 162.6 mAh g−1 was observed for the Ag-1D α-LiFeO2 electrode, with a capacity retention of 89.6% after 50 cycles. Coulombic efficiencies close to 100% (except for the first cycle) were observed in both samples, indicating a superior reversible capacity (Fig. 7c). This initially low coloumbic efficiency has been observed in other Li-Fe–O systems [6, 42, 44]. For reference, the electrochemical performance of the 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes was tested against as-prepared pristine (P-α-LiFeO2) using the same voltage window at 0.1 C (Fig. 7d). As summarized in Table 1, the capacity of P-α-LiFeO2 fades from 176 to 109 mAh g−1 after 50 cycles with only a 61.9% capacity retention, while this is 82.2% and 93.7% for 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes, respectively.
The rate performance of the P-α-LiFeO2, 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes was tested and waterfall plots are shown in Fig. 7e. Samples were cycled at 0.1 C, 0.2 C, 0.5 C, 1 C and 2 C (five cycles each) between 1.5 and 4.8 V and then returned to 0.1 C. Overall, the discharge capacity decreases for all samples with increasing current. The Ag-1D α-LiFeO2 sample delivers the highest of all current densities compared to the other two samples. Average discharge capacities of 193.5, 176.7, 147.9, 115 and 81.18 mAh g−1 at 0.1, 0.2, 0.5, 1 and 2 C were observed for the Ag-1D α-LiFeO2 electrode, which contrast to the average discharge capacities of 176.6 (0.1 C), 167.6 (0.2 C), 133.8 (0.5 C), 94.6 (1 C) and 70.5 mAh g−1 (2 C) for 1D α-LiFeO2 and 175.9 (0.1 C), 127.4 (0.2 C), 109.38 (0.5 C), 73.88 (1 C) and 44.2 mAh g−1 (2 C) for the P-α-LiFeO2 electrode (Fig. 7f). When the current density returns from 2 to 0.1 C, the average discharge capacities of the P-α-LiFeO2, 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes are restored to 97.6, 104.83 and 138 mAh g−1, respectively. We attribute the enhanced rate performance of the Ag-1D α-LiFeO2 sample to the incorporation of Ag NPs on the surface of the nanorods, which allow for improved electronic and ionic conductivity in these materials. This is further confirmed by the impedance analysis (Fig. 8).
To further investigate the effects of Ag on the long cycling performance, 1D α-LiFeO2 and Ag-1D α-LiFeO2 were cycled 100 times at a 0.1 C rate (Fig. S5). Our data show that the capacity retention ratio of 1D α-LiFeO2 and Ag-1D α-LiFeO2 are 49% and 75.93%, respectively. We attribute this improvement in capacity retention to the improved electronic and ionic conductivity in Ag-1D α-LiFeO2.
The Nyquist plots and equivalent circuit of the P-α-LiFeO2, 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes before and after cycling are shown in Fig. S6 and Fig. 8a. The semicircle dimensions of all cells increase after 50 cycles, suggesting an increased charge transfer resistance of the electrodes with cycling [45]. A broader semicircle was observed for the P-α-LiFeO2 electrode before and after 50 cycles compared to the 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes. This is also supported by the fitted parameters listed in Tables S1 and Table 2. The electronic conductivity of the 1D α-LiFeO2 and Ag-1D α-LiFeO2 electrodes was measured, and the results are shown in Table S2. These indicate that the homogeneous incorporation of the silver nanoparticles can decrease the resistance value in the α-LiFeO2 nanorods, providing a long charge carrier transportation length. Furthermore, we calculated the lithium-ion diffusion coefficient of Ag-1D α-LiFeO2 to be 8.24 × 10−12 cm2 S−1, which is about 6 times higher than that for 1D α-LiFeO2 (1.3 × 10−12). See supplementary information for more details.
Given the reduction of the charge transfer resistance value (RCT) and the increase of exchange current density \(\left( {i^{0} } \right)\) after the modification of Ag nanoparticles, it can be concluded that 1D α-LiFeO2 combined with acetylene black to enhance the surface electron conductivity of the electrode, provides more active sites for electron and lithium-ion transfer (Fig. 9). Moreover, the introduction of Ag NPs further enhances the electron conductivity and lithium-ion diffusion coefficient, thereby contributing to the high cycling stability and rate capability.
To understand the structural stability of the Ag-1D α-LiFeO2 nanorods, the morphology of Ag-1D α-LiFeO2 after 50 cycles was investigated by SEM analysis (Fig. S7). It was observed that the Ag nanoparticles still adhere tightly to the 1D α-LiFeO2 nanorods even after 50 cycles at 0.5 C. This indicates enhanced structural stability achieved for the deposition of Ag nanoparticles on the 1 D α-LiFeO2 nanorods via LBL method.
Conclusion
In this work, we report a novel electrostatic layer-by-layer self-assembly method using PAH and PSS charged polyelectrolytes to grow in situ Ag nanoparticles on the surface of α-LiFeO2 nanorods. When tested as a cathode material for LIBs, the Ag-modified 1D α-LiFeO2 nanorods delivered a highly reversible cycling capacity of 194.5 mAh g−1 at 0.1 C after 50 cycles and show an excellent capacity at higher rates (144.8 mAh g−1 at 0.5 C after 50 cycles) with a capacity retention of ca. 94% and 90%, respectively. By contrast, the unmodified 1D α-LiFeO2 sample showed a capacity retention of ca. 82% and 74% under identical conditions. Based on our data, the enhanced rate capability and improved cycling performance of Ag-1D α-LiFeO2 can be ascribed to: (1) synergistic effects by combining Ag NPs with 1D microstructures, where the nanorod microstructure provides facilitated lithium and electron pathways given their large specific surface areas, and the evenly coated Ag NPson the surface of these improve the electronic conductivity (8.2 × 10−9 S cm−1) of the sample given their high electrical conductivity; (2) the large Li+ diffusion coefficient of 8.24 × 10−12 and high exchange current density of 2.791 × 10−4 mA cm−2 observed; and (3) the excellent structural stability upon long cycling without undergoing severe agglomeration.
References
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–334
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4301
Sakurai YJ, Arai H, Yamaki HJ (1998) Preparation of electrochemically active α-LiFeO2 at low temperature. Solid State Ion 113:29–34
Lee YS, Sato S, Sun YK, Kobayakawa K, Sato Y (2003) A new type of orthorhombic LiFeO2 with advanced battery performance and its structural change during cycling. J Power Sources 119:285–289
Kanno R, Shirane T, Inaba Y, Kawamoto Y (1997) Synthesis and electrochemical properties of lithium iron oxides with layer-related structures. J Power Sources 68:145–152
Ado K, Tabuchi M, Kobayashi H, Kageyama H, Nakamura O (1997) Preparation of LiFeO2 with alpha-NaFeO2-type structure using a mixed-alkaline hydrothermal method. J Electrochem Soc 144:177–180
Hu Y, Zhao H, Liu X (2018) A simple, quick and eco-friendly strategy of synthesis nanosized alpha-LiFeO2 cathode with excellent electrochemical performance for lithium-ion batteries. Materials 11:1176
Liu H, Ji P, Han X (2016) Rheological phase synthesis of nanosized α-LiFeO2 with higher crystallinity degree for cathode material of lithium-ion batteries. Mater Chem Phys 183:152–157
Bruce PG, Scrosati B, Tarascon JM (2008) Nanomaterials for rechargeable lithium batteries. Angew Chem 47:2930–2946
Lu YX, Du SF, Wilckens RS (2018) Evolution of gas diffusion layer structures for aligned Pt nanowire electrodes in PEMFC applications. Electrochim Acta 279:99–107
Liang K, He H, Ren Y, Wang H, Liao Y, Huang X (2019) Porous lithium titanate nanosheets as an advanced anode material for sodium ion batteries. J Mater Sci 55:4372–4381. https://doi.org/10.1007/s10853-019-04290-1
Yang Z, Li Z, Li P, Gao C, Zhang H (2019) NiO/Ni nanocomposites embedded in 3D porous carbon with high performance for lithium-ion storage. J Mater Sci 55:1659–1672. https://doi.org/10.1007/s10853-019-04075-6
Li P, Deng J, Li J, Zeng M, Wang L, Guo J (2019) Multifunctional hollow spheres as sulfur hosts for high-performance Li–S batteries. J Mater Sci 55:3964–3973. https://doi.org/10.1007/s10853-019-04251-8
Rahman MM, Wang JZ, Hassan MF, Chou SL, Chen ZX, Liu HK (2011) Nanocrystalline porous α-LiFeO2–C composite: an environmentally friendly cathode for the lithium-ion battery. Energy Environ Sci 4:952–957
Rahman MM, Glushenkov AM, Chen ZQ, Dai XJ, Ramireddy T, Chen Y (2013) Clusters of alpha-LiFeO2 nanoparticles incorporated into multi-walled carbon nanotubes: a lithium-ion battery cathode with enhanced lithium storage properties. Phys Chem Chem Phys 15:20371–20378
Hu Y, Zhao H, Tan M, Liu J, Shu X, Zhang M, Liu S, Ran Q, Li H, Liu X (2019) Synthesis of α-LiFeO2/graphene nanocomposite via layer by layer self-assembly strategy for lithium-ion batteries with excellent electrochemical performance. J Mater Sci Technol. https://doi.org/10.1016/j.jmst.2019.04.044
Guo SP, Ma Z, Li JC, Xue HG (2017) Facile preparation and promising lithium storage ability of α-LiFeO2/porous carbon nanocomposite. J Alloys Compd 711:8–14
Kalantarian MM, Asgari S, Mustarelli P (2014) A theoretical approach to evaluate the rate capability of Li-ion battery cathode materials. J Mater Chem A 2:107–115
Li Y, Zhou X, Bai Y, Chen G, Wang Z, Li H, Wu F, Wu C (2017) Building an electronic bridge via Ag decoration to enhance kinetics of iron fluoride cathode in lithium-ion batteries. ACS Appl Mater Interfaces 9:19852–19860
Zhang H, Zong P, Chen M, Jin H, Bai Y, Li S, Ma F, Xu H, Lian K (2019) In situ synthesis of multilayer carbon matrix decorated with copper particles: enhancing the performance of Si as Anode for Li-ion batteries. ACS Nano 13:3054–3062
Lin XT, Wang PF, Li P, Yu HX, Qian SS, Shui M, Wang DJ, Long NB, Shu J (2015) Improved the lithium storage capability of BaLi2Ti6O14 by electroless silver coating. Electrochim Acta 186:24–33
Kim SH, Choi SY (2015) Fabrication of Cu-coated TiO2 nanotubes and enhanced electrochemical performance of lithium ion batteries. J Electroanal Chem 744:45–52
Zhou WJ, He BL, Li HL (2008) Synthesis, structure and electrochemistry of Ag-modified LiMn2O4 cathode materials for lithium-ion batteries. Mater Res Bull 43:2285–2294
Parka KS, Sona JT, Kim SJ, Chung HT, Lee CH, Kang KT, Kim HG (2004) Surface modification by silver coating for improving electrochemical properties of LiFePO4. Solid State Commun 129:311–314
Hao Q, Yu Y, Zhao D, Xu C (2015) Composited Co3O4/Ag with flower-like nanosheets anchored on a porous substrate as a high-performance anode for Li-ion batteries. J Mater Chem A 3:15944–15950
Yin SS, Zhao D, Ji Q, Xia Y, Xia S, Wang X, Wang M, Ban J, Zhang Y, Metwalli E, Wang X, Xiao Y, Zuo X, Xie S, Fang K, Liang S, Zheng L, Qiu B, Yang Z, Lin Y, Chen L, Wang C, Liu Z, Zhu J, Muller-Buschbaum P, Cheng YJ (2018) Si/Ag/C nanohybrids with in situ incorporation of super-small silver nanoparticles: tiny amount, huge impact. ACS Nano 12:861–875
Li Y, Li W, Luo Y, Fang M, Yao X, Shui M, Shu J, Ren Y (2018) The enhanced electrochemical performance of Ag coating Kx Cu1−x O1−γ as high performance anode materials for lithium-ion batteries. J Electroanal Chem 818:114–123
Han X, Huang Y, Gao Q, Yu M, Chen X (2018) High thermal conductivity and mechanical properties of nanotube@Cu/Ag@graphite/aluminum composites. Ind Eng Chem Res 57:10365–10371
Cheong JY, Youn DY, Kim C, Jung JW, Ogata AF, Bae JG, Kim ID (2018) Ag-coated one-dimensional orthorhombic Nb2O5 fibers as high performance electrodes for lithium storage. Electrochim Acta 269:388–396
Xiang Y, Lu S, Jiang SP (2012) Layer-by-layer self-assembly in the development of electrochemical energy conversion and storage devices from fuel cells to supercapacitors. Chem Soc Rev 41:7291–7321
Richardson JJ, Bjornmalm M, Caruso F (2015) Technology-driven layer-by-layer assembly of nanofilms. Science 348:2491–2501
Zhai Y, Xu L, Qian Y (2016) Ce-doped α-FeOOH nanorods as high-performance anode material for energy storage. J Power Sources 327:423–431
Tian GY, Scheib F, Pfaffmann L, Fiedler A, Chakravadhanula VSK, Balachandran G, Zhao ZJ, Ehrenberg H (2018) Electrostatic self-assembly of LiFePO4 cathodes on a three-dimensional substrate for lithium ion batteries. Electrochim Acta 283:1375–1383
Wang X, Gao LS, Zhou F, Zhang ZD, Ji MR, Tang CM, Shen T, Zheng HG (2004) Large-scale synthesis of α-LiFeO2 nanorods by low-temperature molten salt synthesis (MSS) method. J Cryst Growth 265:220–223
Meng E, Zhang M, Hu Y, Gong F, Zhang LS, Li F (2018) Solid-state attachments of Ag nanoparticles onto the surfaces of LiFePO4 cathode materials for Li storage with enhanced capabilities. Electrochim Acta 265:160–165
Sahoo PK, Kumar N, Thiyagarajan S, Thakur D, Panda HS (2018) Freeze-casting of multifunctional cellular 3D-graphene/Ag nanocomposites: synergistically affect supercapacitor, catalytic, and antibacterial properties. ACS Sustain Chem Eng 6:7475–7487
Shilpa, Sharma A (2017) Template-free synthesis of hollow Li2O–Fe2O3–Ag heterostructures for ultra-high performance Li-ion batteries. J Mater Chem A 5:14220–14229
Hao Q, Yu Y, Zhao DY, Xu CX (2015) Composited Co3O4/Ag with flower-like nanosheets anchored on a porous substrate as a highperformance anode for Li-ion batteries. J Mater Chem A 3:15944–15950
Wu SH, Liu HY (2007) Low temperature synthesis and electrochemical characteristics of LiFeO2 cathodes. J Power Sources 174:789–794
Wang Y, Wang J, Liao H, Qian X, Zhu Y, Cheng S (2013) Synthesis and characterization of nanosized α-LiFeO2 with increased electrochemical activity. Int J Electrochem Sci 8:8730–8739
Sakurai Y, Arai H, Okada S, Yamaki J (1997) Preparation of electrochemically active α-LiFeO2 at low temperature, low temperature synthesis and electrochemical characteristics of LiFeO2 cathodes. J Power Sources 68:711–715
Morales J, Santos-Peña J (2007) Highly electroactive nanosized α-LiFeO2. Electrochem Commun 9:2116–2120
Kanno R, Shirane T, Kawamoto Y (1996) Synthesis, structure, and electrochemical properties of a new lithium iron oxide, LiFeO2, with a corrugated layer structure. J Electrochem Soc 8:2435–2441
Yu YL, Li G, Zhou S, Chen X, Lee HW, Yang WS (2017) Self-adaptive Si/reduced graphene oxide scrolls for high-performance Li-ion battery anodes. Carbon 120:397–404
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21071026) and the Outstanding Talent Introduction Project of University of Electronic Science and Technology of China (No. 08JC00303). NTR would like to acknowledge the UK-China Research and Innovation Partnership Fund to provide YH with a scholarship to conduct research in her laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Y., Liu, X. & Tapia-Ruiz, N. Electrostatic layer-by-layer self-assembly of 1D α-LiFeO2 with enhanced rate capability and cycling performance. J Mater Sci 55, 8651–8664 (2020). https://doi.org/10.1007/s10853-020-04581-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04581-y