Abstract
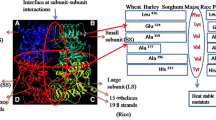
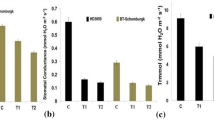
ADP-glucose pyrophosphorylase (AGPase) activity in the developing grains of two contrasting wheat cultivars WH730 (thermo-tolerant) and UP2565 (thermo-sensitive) was determined in relation to their allosteric effectors and grain growth. The developing grains (35 days after anthesis) were excised from the middle portion of spikes of wheat genotypes subjected to high temperature, drought and their combination at booting, post-anthesis and booting+post-anthesis. The impact of stress treatments was studied by measuring starch content and yield attributes in relation to AGPase activity. AGPase, a key enzyme for starch synthesis, is allosterically activated by 3-phosphoglyceric acid (3-PGA) and inhibited by inorganic phosphate (Pi). Sensitivity of AGPase towards individual and combined high temperature and drought has not been adequately investigated, therefore the present study analyzed AGPase activity, its sensitivity to allosteric effectors under influence of high temperature, drought in order to elucidate the relationship of AGPase with starch accumulation and grain growth. Significant difference in behavior of the enzyme and its allosteric effectors were observed between the two cultivars under high temperature and/or drought. AGPase activity was substantially decreased by high temperature, drought and was found to be positively correlated with the 3-PGA, starch accumulation and yield attributes, while negatively correlated with Pi content. The results showed that effects of high temperature and drought were additive and more severe at booting+post-anthesis stage. Such studies might help in understanding the control mechanisms associated with the pathway of starch biosynthesis and thus provide chemical means to manipulate starch content vis-à-vis grain yield under heat and drought stress.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- 3-PGA:
-
3-phosphoglycerate
- Pi:
-
inorganic phosphate
- HT:
-
high temperature
- D:
-
drought
- HT+D:
-
high temperature along with drought
- DAA:
-
days after anthesis
- DAS:
-
days after sowing
- PWP:
-
permanent wilting point
- RH:
-
relative humidity
References
Ahmadi, A., Baker, D.A. 2001. The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul. 35:81–91.
Ballicora, M.A., Iglesias, A.A., Priess, J. 2004. ADP-glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynth. Res. 79:1–24.
Boehlein, S.K., Shaw, J.R., Georgelis, N., Hannah, L.C. 2014. Enhanced heat stability and kinetic parameters of maize endosperm ADP-glucose pyrophosphorylase by alteration of phylogenetically identified amino acids. Arch. Biochem. Biophys. 543:1–9.
Clegg, K.M. 1956. The application of anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agr. 7:40–44.
Doan, D.N.P., Rudi, H., Olsen, O.A. 1999. The allosterically unregulated isoform of ADP-glucose pyrophosphorylase from barley endosperm is the most likely sources of ADP-glucose incorporated into endosperm starch. Plant Physiol. 121:965–975.
Geigenkerger, P., Gerger, M., Stitt, M. 1998. High temperature perturbation of starch synthesis is attributable to inhibition of AGPase by decreased levels of glycerate 3-phosphate in growing potato tubers. Plant Physiol. 117:1307–1316.
Gómez-Casati, D.F., Iglesias, A.A. 2002. ADP-glucose pyrophosphorylase from wheat endosperm. Purification and characterization of an enzyme with novel regulatory properties. Planta 214:428–434.
Heldt, H.W., Chon, C.J., Maronde, D., Herold, A., Stankovic, Z.S., Walker, D., Kraminer, A., Kirk, M.R., Heber, U. 1977. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 59:1146–1155.
Kaur, V., Behl, R.K. 2010. Grain yield in wheat as affected by short periods of high temperature, drought and their interaction during pre- and post-anthesis stages. Cereal Res. Commun. 38:514–520.
Kaur, V., Mahla, R., Behl, R.K. 2014. High temperature, drought and their interaction induced protein alterations in sensitive and tolerant wheat varieties. Electron. J. Plant Breed. 5:641–650.
Kleczkowski, L.A., Villand, P., Luthi, E. Olsen, O.A., Preiss, J. 1993. Insensitivity of barley endosperm ADP-glucose pyrophosphorylase to 3-phosphoglycerate and orthophosphate regulation. Plant Physiol. 101:179–186.
Latzko, E., Gibbs, M. 1972. Measurements of intermediates of photosynthetic carbon reduction cycle using enzymatic method. In: Pietro, A.S. (ed.), Methods of Enzymology. Academic Press. New York, USA. pp. 261–268.
Lohot, V.D., Sharma-Natu, P., Pandey, R., Ghildiyal, M.C. 2010. ADP-glucose pyrophosphorylase activity in relation to starch accumulation and grain growth in wheat cultivars. Curr. Sci. 98:426–430.
Ozbun, J.L., Hawker, J.S., Greenberg, E., Lamelli, C., Priess, J. 1973. Starch synthase phosphorylase, ADP-glucose pyrophosphorylase and UDP-glucopyrophosphorylase in developing maize kernels. Plant Physiol. 51:1–9.
Preiss, J., Hutney, J., Smith-White, B., Li, L., Okita, T.W. 1991. Regulatory mechanisms involved in the biosynthesis of starch. J. Pure Appl. Chem. 63:535–544.
Rane, J., Nagarajan, S. 2004. High temperature index for field evaluation of heat tolerance in wheat varieties. Agr. Syst. 79:243–255.
Sakulsingharoj, C., Choi, S.B., Hwang, S.K., Edwards, G.E., Bork, J., Meyer, C.R., Preiss, J., Okita, T.W. 2004. Engineering starch biosynthesis for increasing rice seed weight: the role of the cytoplasmic ADP-glucose pyrophosphorylase. Plant Sci. 167:1323–1333.
SAS 2011. The SAS system for Windows. Release 9.2., SAS Institute, Cary, NC, USA.
Savitch, L.V., Harney, T., Huner, N.P.A. 2000. Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimatization. Physiol. Plant. 108:270–278.
Sheikh, S., Sikka, V.K., Behl, R.K., Kumar, A. 2010. Grain growth rate and grain yield in relation to ADP-glucose pyrophosphorylase activity in wheat (Triticum aestivumL. em. Thell) under normaland late sown conditions. Cereal Res. Commun. 38:589–599.
Sikka, V.K., Choi, S.B., Kavakli, I.H., Sakulsingharoj, C., Gupta, S., Ito, H., Okita, T.W. 2001. Subcellular compartmentation and allosteric regulation of the rice endosperm ADP-glucose pyrophosphorylase. Plant Sci. 161:461–468.
Slattery, C.J., Kavakli, H.I., Okita, T.W. 2004. Engineering starch for increased quantity and quality. Trends Plant Sci. 5:291–298.
Smidansky, E.D., Clancy, M., Meyer, F.D., Lanning, S.P., Blake, N.K., Talbert, L.E., Giroux, M.J. 2002. Enhanced AGPase activity in wheat endosperm increases seed yield. Proc. Natl. Acad. Sci. 99:1724–1729.
Smidansky, E.D., Martin, J.M., Hannah, L.C., Fisher, A.M., Giroux, M.J. 2003. Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216:656–664.
Sowokinos, J.R., Preiss, J. 1982. Pyrophosphorylases in Solanum tuberosumIII. Purification, physical and catalytic properties of ADP-glucosepyrophosphorylase in potatoes. Plant Physiol. 69:1459–1466.
Stark, D.M., Timmerman, K.P., Barry, G.F., Preiss, J., Kishore, G.M. 1992. Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science 258:287–292.
Stitt, M. 1990. Fructose 2, 6 bisphosphate. In: Leo, P.J. (ed.), Methods in Plant Biochemistry. Academic Press, New York, pp. 87–92.
Stitt, M. 1996. Metabolic regulation of photosynthesis. In: Baker, N.R. (ed), Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 151–191.
Sumesh, K.V., Sharma-Natu, P., Ghildiyal, M.C. 2008. Starch synthase activity and heat shock protein in relation to thermal tolerance of developing wheat grains. Biol. Plant 52:749–753.
Wang, Z., Chen, X., Wang, J., Liu, T., Liu, Y., Zhao, L., Wang, G. 2007. Increasing maize seed weight by enhancing the cytoplasmic ADP-glucose pyrophosphorylase activity in transgenic plants. Plant Cell Tissue Organ Cult. 88:83–92.
Yan, S.H., Yin, Y.P., Li, W.Y., Li, Y., Liang, T.B., Wu, Y.H., Geng, Q.H., Wang, Z.L. 2008. Effect of high temperature after anthesis on starch formation of two wheat cultivars differing in heat tolerance. Acta Ecologica Sinica 28:6138–6147.
Yang, J., Zhang, J., Wang, Z., Xu, G., Zhu, Q. 2004. Activities of key enzymes in sucrose to starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 135:1621–1629.
Zahedi, M., Sharma, R., Jenner, C.F. 2003. Effect of high temperature on grain growth, metabolites and enzymes of starch synthesis pathway in grains of two wheat cultivars differing in their response to temperature. Funct. Plant Biol. 30:291–300.
Zhao, H., Dai, T., Jing, Q., Jiang, D., Cao, W. 2007. Leaf senescence and grain filling affects by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul. 51:149–158.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Molnár-Láng
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kaur, V., Madaan, S. & Behl, R.K. ADP-glucose Pyrophosphorylase Activity in Relation to Yield Potential of Wheat: Response to Independent and Combined High Temperature and Drought Stress. CEREAL RESEARCH COMMUNICATIONS 45, 181–191 (2017). https://doi.org/10.1556/0806.45.2017.003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/0806.45.2017.003