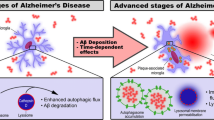
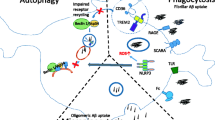
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive cognitive decline, amyloid-β (Aβ) plaques and the formation of neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau. Increasing evidence has demonstrated that the damage of cell plays an important role in AD. Cell death is a critical phenomenon for physiological functions, which promotes AD pathogenesis. Programmed cell death, including necroptosis, pyroptosis, autophagy, and ferroptosis, have been discovered that have unique biological functions and pathophysiological characteristics. Here, we review the available evidence detailing the mechanisms of programmed microglial death, including pyroptosis, autophagy, and ferroptosis. We also highlight the role of programmed death of microglia during the process of AD and focus on the connection between the disease and cell death.



Similar content being viewed by others
References
DeTure, M.A. and D.W. Dickson, The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener, 2019. 14(1): p. 32. doi:https://doi.org/10.1186/s13024-019-0333-5
2020 Alzheimer’s disease facts and figures. Alzheimers Dement, 2020. doi:https://doi.org/10.1002/alz.12068
Mullane, K. and M. Williams, Alzheimer’s disease beyond amyloid: Can the repetitive failures of amyloid-targeted therapeutics inform future approaches to dementia drug discovery? Biochem Pharmacol, 2020. 177: p. 113945. doi:https://doi.org/10.1016/j.bcp.2020.113945
Sharma, I., et al., Exploring the Focal Role of Pyroptosis in Diabetes Mellitus. Biointerface Research in Applied Chemistry, 2021. 11(5): p. 13557–13572. doi:https://doi.org/10.33263/briac115.1355713572
Vanden Berghe, T., et al., Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol, 2014. 15(2): p. 135–47. doi:https://doi.org/10.1038/nrm3737
Yang, W.S. and B.R. Stockwell, Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol, 2016. 26(3): p. 165–176. doi:https://doi.org/10.1016/j.tcb.2015.10.014
D’Arcy, M.S., Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int, 2019. 43(6): p. 582–592. doi:https://doi.org/10.1002/cbin.11137
Yan, N. and J. Zhang, Iron Metabolism, Ferroptosis, and the Links With Alzheimer’s Disease. Front Neurosci, 2019. 13: p. 1443. doi:https://doi.org/10.3389/fnins.2019.01443
Li, Q., Y. Liu, and M. Sun, Autophagy and Alzheimer’s Disease. Cell Mol Neurobiol, 2017. 37(3): p. 377–388. doi:https://doi.org/10.1007/s10571-016-0386-8
Li, Y., et al., Interaction between hyperphosphorylated tau and pyroptosis in forskolin and streptozotocin induced AD models. Biomed Pharmacother, 2020. 121: p. 109618. doi:https://doi.org/10.1016/j.biopha.2019.109618
Louveau, A. [Cerebral lymphatic drainage: implication in the brain immune privilege]. Med Sci (Paris), 2015. 31(11): p. 953–6. doi:https://doi.org/10.1051/medsci/20153111005
Sevenich, L., Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front Immunol, 2018. 9: p. 697. doi:https://doi.org/10.3389/fimmu.2018.00697
Singh, V., et al., Isolation and Characterization of Microglia from Adult Mouse Brain: Selected Applications for ex Vivo Evaluation of Immunotoxicological Alterations Following in Vivo Xenobiotic Exposure. Chemical Research in Toxicology, 2014. 27(5): p. 895–903. doi:https://doi.org/10.1021/tx500046k
Amor, S., et al., Inflammation in neurodegenerative diseases. Immunology, 2010. 129(2): p. 154–69. doi:https://doi.org/10.1111/j.1365-2567.2009.03225.x
Zhang, J., et al., Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy. Life Sci, 2021. 276: p. 118957. doi:https://doi.org/10.1016/j.lfs.2020.118957
Russell, R.C., H.X. Yuan, and K.L. Guan, Autophagy regulation by nutrient signaling. Cell Res, 2014. 24(1): p. 42–57. doi:https://doi.org/10.1038/cr.2013.166
Jung, S., H. Jeong, and S.W. Yu, Autophagy as a decisive process for cell death. Exp Mol Med, 2020. 52(6): p. 921–930. doi:https://doi.org/10.1038/s12276-020-0455-4
Wang, Y. and W.D. Le, Autophagy and Ubiquitin-Proteasome System. Adv Exp Med Biol, 2019. 1206: p. 527–550. doi:https://doi.org/10.1007/978-981-15-0602-4_25
Li, M., et al., Autophagy in the HTR-8/SVneo Cell Oxidative Stress Model Is Associated with the NLRP1 Inflammasome. Oxid Med Cell Longev, 2021. 2021: p. 2353504. doi:https://doi.org/10.1155/2021/2353504
Ip, W.K.E., et al., Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science, 2017. 356(6337): p. 513–519. doi:https://doi.org/10.1126/science.aal3535
Qiu, P., Y. Liu, and J. Zhang, Review: the Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation, 2019. 42(1): p. 6–19. doi:https://doi.org/10.1007/s10753-018-0890-8
Wu, A.G., et al., Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res Rev, 2021. 65: p. 101202. doi:https://doi.org/10.1016/j.arr.2020.101202
Ma, K., et al., Toll-Like Receptor 2-Mediated Autophagy Promotes Microglial Cell Death by Modulating the Microglial M1/M2 Phenotype. Inflammation, 2020. 43(2): p. 701–711. doi:https://doi.org/10.1007/s10753-019-01152-5
Gordon, R., et al., Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med, 2018. 10(465). doi:https://doi.org/10.1126/sdtranslmed.aah4066
Ising, C., et al., NLRP3 inflammasome activation drives tau pathology. Nature, 2019. 575(7784): p. 669–673. doi:https://doi.org/10.1038/s41586-019-1769-z
Han, H.E., et al., Activation of Autophagy Pathway Suppresses the Expression of iNOS, IL6 and Cell Death of LPS-Stimulated Microglia Cells. Biomol Ther (Seoul), 2013. 21(1): p. 21–8. doi:https://doi.org/10.4062/biomolther.2012.089
Cho, M.H., et al., Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy, 2014. 10(10): p. 1761–75. doi:https://doi.org/10.4161/auto.29647
Su, P., et al., The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience, 2016. 319: p. 155–67. doi:https://doi.org/10.1016/j.neuroscience.2016.01.035
Reddy, P.H. and D.M. Oliver, Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells, 2019. 8(5). doi:https://doi.org/10.3390/cells8050488
Reddy, P.H., Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem, 2006. 96(1): p. 1–13. doi:https://doi.org/10.1111/j.1471-4159.2005.03530.x
Thellung, S., et al., Pharmacological activation of autophagy favors the clearing of intracellular aggregates of misfolded prion protein peptide to prevent neuronal death. Cell Death Dis, 2018. 9(2): p. 166. doi:https://doi.org/10.1038/s41419-017-0252-8
Tsvetkov, A.S., et al., A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci U S A, 2010. 107(39): p. 16982–7. doi:https://doi.org/10.1073/pnas.1004498107
Kovács, T., et al., The small molecule AUTEN-99 (autophagy enhancer-99) prevents the progression of neurodegenerative symptoms. Sci Rep, 2017. 7: p. 42014. doi:https://doi.org/10.1038/srep42014
Nilsson, P. and T.C. Saido, Dual roles for autophagy: degradation and secretion of Alzheimer’s disease Aβ peptide. Bioessays, 2014. 36(6): p. 570–8. doi:https://doi.org/10.1002/bies.201400002
Wani, A., et al., Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy, 2021: p. 1–20. doi:https://doi.org/10.1080/15548627.2021.1872187
Glatigny, M., et al., Autophagy Is Required for Memory Formation and Reverses Age-Related Memory Decline. Curr Biol, 2019. 29(3): p. 435–448.e8. doi:https://doi.org/10.1016/j.cub.2018.12.021
Majumder, S., et al., Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One, 2011. 6(9): p. e25416. doi:https://doi.org/10.1371/journal.pone.0025416
Spilman, P., et al., Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One, 2010. 5(4): p. e9979. doi:https://doi.org/10.1371/journal.pone.0009979
Zhu, Z., et al., Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both β-amyloid production and clearance. J Neurosci, 2013. 33(32): p. 13138–49. doi:https://doi.org/10.1523/jneurosci.4790-12.2013
Lonskaya, I., et al., Diminished parkin solubility and co-localization with intraneuronal amyloid-β are associated with autophagic defects in Alzheimer’s disease. J Alzheimers Dis, 2013. 33(1): p. 231–47. doi:https://doi.org/10.3233/jad-2012-121141
Cai, Q. and Y.Y. Jeong, Mitophagy in Alzheimer’s Disease and Other Age-Related Neurodegenerative Diseases. Cells, 2020. 9(1). doi:https://doi.org/10.3390/cells9010150
Pradeepkiran, J.A., A.P. Reddy, and P.H. Reddy, Pharmacophore-based models for therapeutic drugs against phosphorylated tau in Alzheimer’s disease. Drug Discov Today, 2019. 24(2): p. 616–623. doi:https://doi.org/10.1016/j.drudis.2018.11.005
Oliver, D. and P.H. Reddy, Dynamics of Dynamin-Related Protein 1 in Alzheimer’s Disease and Other Neurodegenerative Diseases. Cells, 2019. 8(9). doi:https://doi.org/10.3390/cells8090961
Menzies, F.M., et al., Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron, 2017. 93(5): p. 1015–1034. doi:https://doi.org/10.1016/j.neuron.2017.01.022
Kang, S., et al., Autophagy-Mediated Secretory Pathway is Responsible for Both Normal and Pathological Tau in Neurons. J Alzheimers Dis, 2019. 70(3): p. 667–680. doi:https://doi.org/10.3233/jad-190180
Kandimalla, R., et al., Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum Mol Genet, 2018. 27(1): p. 30–40. doi:https://doi.org/10.1093/hmg/ddx381
Manczak, M. and P.H. Reddy, Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet, 2012. 21(11): p. 2538–47. doi:https://doi.org/10.1093/hmg/dds072
Manczak, M. and P.H. Reddy, Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum Mol Genet, 2012. 21(23): p. 5131–46. doi:https://doi.org/10.1093/hmg/dds360
Di Meco, A., et al., Autophagy Dysfunction in Alzheimer’s Disease: Mechanistic Insights and New Therapeutic Opportunities. Biol Psychiatry, 2020. 87(9): p. 797–807. doi:https://doi.org/10.1016/j.biopsych.2019.05.008
Collier, J.J., et al., Developmental Consequences of Defective ATG7-Mediated Autophagy in Humans. N Engl J Med, 2021. 384(25): p. 2406–2417. doi:https://doi.org/10.1056/NEJMoa1915722
Zhang, Z., et al., Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res Rev, 2021. 72: p. 101464. doi:https://doi.org/10.1016/j.arr.2021.101464
Estfanous, S., et al., Elevated Expression of MiR-17 in Microglia of Alzheimer’s Disease Patients Abrogates Autophagy-Mediated Amyloid-β Degradation. Front Immunol, 2021. 12: p. 705581. doi:https://doi.org/10.3389/fimmu.2021.705581
Dixon, S.J., et al., Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 2012. 149(5): p. 1060–72. doi:https://doi.org/10.1016/j.cell.2012.03.042
Gao, M., et al., Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell, 2015. 59(2): p. 298–308. doi:https://doi.org/10.1016/j.molcel.2015.06.011
Stockwell, B.R., et al., Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell, 2017. 171(2): p. 273–285. doi:https://doi.org/10.1016/j.cell.2017.09.021
Cao, J.Y. and S.J. Dixon, Mechanisms of ferroptosis. Cell Mol Life Sci, 2016. 73(11–12): p. 2195–209. doi:https://doi.org/10.1007/s00018-016-2194-1
Fanzani, A. and M. Poli, Iron, Oxidative Damage and Ferroptosis in Rhabdomyosarcoma. Int J Mol Sci, 2017. 18(8). doi:https://doi.org/10.3390/ijms18081718
Youssef, L.A., et al., Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood, 2018. 131(23): p. 2581–2593. doi:https://doi.org/10.1182/blood-2017-12-822619
Yao, M.Y., et al., Role of ferroptosis in neurological diseases. Neurosci Lett, 2021. 747: p. 135614. doi:https://doi.org/10.1016/j.neulet.2020.135614
Yang, W.S., et al., Regulation of ferroptotic cancer cell death by GPX4. Cell, 2014. 156(1–2): p. 317–331. doi:https://doi.org/10.1016/j.cell.2013.12.010
Dixon, S.J. and B.R. Stockwell, The Hallmarks of Ferroptosis. Annual Review of Cancer Biology, 2019. 3(1): p. 35–54. doi:https://doi.org/10.1146/annurevcancerbio-030518-055844
Luo, X., et al., Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ, 2021. 28(6): p. 1971–1989. doi:https://doi.org/10.1038/s41418-020-00719-2
Seibt, T.M., B. Proneth, and M. Conrad, Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med, 2019. 133: p. 144–152. doi:https://doi.org/10.1016/j.freeradbiomed.2018.09.014
Kagan, V.E., et al., Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol, 2017. 13(1): p. 81–90. doi:https://doi.org/10.1038/nchembio.2238
Dar, H.H., et al., A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(») sabotage of theftferroptosis. Redox Biol, 2021. 45: p. 102045. doi:https://doi.org/10.1016/j.redox.2021.102045
Weiland, A., et al., Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol, 2019. 56(7): p. 4880–4893. doi:https://doi.org/10.1007/s12035-018-1403-3
Wu, J.R., Q.Z. Tuo, and P. Lei, Ferroptosis, a Recent Defined Form of Critical Cell Death in Neurological Disorders. J Mol Neurosci, 2018. 66(2): p. 197–206. doi:https://doi.org/10.1007/s12031-018-1155-6
McIntosh, A., et al., Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol, 2019. 29(5): p. 606–621. doi:https://doi.org/10.1111/bpa.12704
Ayton, S., et al., Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry, 2020. 25(11): p. 2932–2941. doi:https://doi.org/10.1038/s41380-019-0375-7
Ghadery, C., et al., R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging, 2015. 36(2): p. 925–32. doi:https://doi.org/10.1016/j.neurobiolaging.2014.09.013
Acosta-Cabronero, J., et al., In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan. J Neurosci, 2016. 36(2): p. 364–74. doi:https://doi.org/10.1523/jneurosci.1907-15.2016
Smith, M.A., et al., Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis, 2010. 19(1): p. 363–72. doi:https://doi.org/10.3233/jad-2010-1239
Ayton, S., N.G. Faux, and A.I. Bush, Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun, 2015. 6: p. 6760. doi:https://doi.org/10.1038/ncomms7760
Kroner, A., et al., TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron, 2014. 83(5): p. 1098–116. doi:https://doi.org/10.1016/j.neuron.2014.07.027
van Duijn, S., et al., Cortical Iron Reflects Severity of Alzheimer’s Disease. J Alzheimers Dis, 2017. 60(4): p. 1533–1545. doi:https://doi.org/10.3233/jad-161143
Ayton, S., et al., Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain, 2017. 140(8): p. 2112–2119. doi:https://doi.org/10.1093/brain/awx137
van Bergen, J.M.G., et al., Simultaneous quantitative susceptibility mapping and Flutemetamol-PET suggests local correlation of iron and β-amyloid as an indicator of cognitive performance at high age. Neuroimage, 2018. 174: p. 308–316. doi:https://doi.org/10.1016/j.neuroimage.2018.03.021
Yan, H.F., et al., Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther, 2021. 6(1): p. 49. doi:https://doi.org/10.1038/s41392-020-00428-9
Fu, A.L., Z.H. Dong, and M.J. Sun, Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res, 2006. 1109(1): p. 201–6. doi:https://doi.org/10.1016/j.brainres.2006.06.042
Bao, W.D., et al., Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ, 2021. 28(5): p. 1548–1562. doi:https://doi.org/10.1038/s41418-020-00685-9
Reichert, C.O., et al., Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int J Mol Sci, 2020. 21(22). doi:https://doi.org/10.3390/ijms21228765
Smith, M.A., et al., Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A, 1997. 94(18): p. 9866–8. doi:https://doi.org/10.1073/pnas.94.18.9866
Svobodová, H., et al., Elevated age-related cortical iron, ferritin and amyloid plaques in APP(swe)/PS1(deltaE9) transgenic mouse model of Alzheimer’s disease. Physiol Res, 2019. 68(Suppl 4): p. S445–S451. doi:https://doi.org/10.33549/physiolres.934383
Nikseresht, S., A.I. Bush, and S. Ayton, Treating Alzheimer’s disease by targeting iron. Br J Pharmacol, 2019. 176(18): p. 3622–3635. doi:https://doi.org/10.1111/bph.14567
Wong, B.X., et al., β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One, 2014. 9(12): p. e114174. doi:https://doi.org/10.1371/journal.pone.0114174
Tuo, Q.Z., et al., Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry, 2017. 22(11): p. 1520–1530. doi:https://doi.org/10.1038/mp.2017.171
Long, H.Z., et al., The Role of Microglia in Alzheimer’s Disease From the Perspective of Immune Inflammation and Iron Metabolism. Front Aging Neurosci, 2022. 14: p. 888989. doi:https://doi.org/10.3389/fnagi.2022.888989
Fernández-Mendívil, C., et al., Aging and Progression of Beta-Amyloid Pathology in Alzheimer’s Disease Correlates with Microglial Heme-Oxygenase-1 Overexpression. Antioxidants (Basel), 2020. 9(7). doi:https://doi.org/10.3390/antiox9070644
Chang, Y., et al., NLRP3 inflammasome-mediated microglial pyroptosis is critically involved in the development of post-cardiac arrest brain injury. J Neuroinflammation, 2020. 17(1): p. 219. doi:https://doi.org/10.1186/s12974-020-01879-1
Man, S.M., R. Karki, and T.D. Kanneganti, Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev, 2017. 277(1): p. 61–75. doi:https://doi.org/10.1111/imr.12534
Jorgensen, I. and E.A. Miao, Pyroptotic cell death defends against intracellular pathogens. Immunol Rev, 2015. 265(1): p. 130–42. doi:https://doi.org/10.1111/imr.12287
Shi, J., W. Gao, and F. Shao, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci, 2017. 42(4): p. 245–254. doi:https://doi.org/10.1016/j.tibs.2016.10.004
Zeng, C.Y., et al., ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages. Apoptosis, 2019. 24(9–10): p. 703–717. doi:https://doi.org/10.1007/s10495-019-01551-x
Bergsbaken, T., S.L. Fink, and B.T. Cookson, Pyroptosis: host cell death and inflammation. Nat Rev Microbiol, 2009. 7(2): p. 99–109. doi:https://doi.org/10.1038/nrmicro2070
Ding, H.G., et al., Hypercapnia promotes microglial pyroptosis via inhibiting mitophagy in hypoxemic adult rats. CNS Neurosci Ther, 2020. 26(11): p. 1134–1146. doi:https://doi.org/10.1111/cns.13435
Poh, L., et al., Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun, 2019. 75: p. 34–47. doi:https://doi.org/10.1016/j.bbi.2018.09.001
Tan, M.S., et al., Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis, 2014. 5(8): p. e1382. doi:https://doi.org/10.1038/cddis.2014.348
Ross, J., et al., A selective, non-peptide caspase-1 inhibitor, VRT-018858, markedly reduces brain damage induced by transient ischemia in the rat. Neuropharmacology, 2007. 53(5): p. 638–42. doi:https://doi.org/10.1016/j.neuropharm.2007.07.015
Heneka, M.T., et al., NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature, 2013. 493(7434): p. 674–8. doi:https://doi.org/10.1038/nature11729
Halle, A., et al., The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol, 2008. 9(8): p. 857–65. doi:https://doi.org/10.1038/ni.1636
Hook, V.Y., M. Kindy, and G. Hook, Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem, 2008. 283(12): p. 7745–53. doi:https://doi.org/10.1074/jbc.M708362200
Chen, H., et al., Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett, 2016. 246: p. 7–16. doi:https://doi.org/10.1016/j.toxlet.2016.01.014
Shen, H., et al., Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain Behav, 2021. 11(4): p. e02063. doi:https://doi.org/10.1002/brb3.2063
Griffin, W.S., et al., Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation, 2006. 3: p. 5. doi:https://doi.org/10.1186/1742-2094-3-5
Biundo, F., et al., A role for tau in learning, memory and synaptic plasticity. Sci Rep, 2018. 8(1): p. 3184. doi:https://doi.org/10.1038/s41598-018-21596-3
Wagner, L.K., et al., Immunoproteasome deficiency alters microglial cytokine response and improves cognitive deficits in Alzheimer’s disease-like APPPS1 mice. Acta Neuropathol Commun, 2017. 5(1): p. 52. doi:https://doi.org/10.1186/s40478-017-0453-5
Sita, G., et al., NLRP3 and Infections: β-Amyloid in Inflammasome beyond Neurodegeneration. Int J Mol Sci, 2021. 22(13). doi:https://doi.org/10.3390/ijms22136984
Litwiniuk, A., et al., Contribution of Mitochondrial Dysfunction Combined with NLRP3 Inflammasome Activation in Selected Neurodegenerative Diseases. Pharmaceuticals (Basel), 2021. 14(12). doi:https://doi.org/10.3390/ph14121221
Sharma, D. and T.D. Kanneganti, The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol, 2016. 213(6): p. 617–29. doi:https://doi.org/10.1083/jcb.201602089
Sharma, B., et al., Role of NLRP3 Inflammasome and Its Inhibitors as Emerging Therapeutic Drug Candidate for Alzheimer’s Disease: a Review of Mechanism of Activation, Regulation, and Inhibition. Inflammation, 2022: p. 1–32. doi:https://doi.org/10.1007/s10753-022-01730-0
Acknowledgements
This work was supported by the National Natural Science Foundation of China [82001473].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Zhongshan Hospital of Fudan university.
Additional information
Conflict of interest
All authors claim that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Qiu, Z., Zhang, H., Xia, M. et al. Programmed Death of Microglia in Alzheimer’s Disease: Autophagy, Ferroptosis, and Pyroptosis. J Prev Alzheimers Dis 10, 95–103 (2023). https://doi.org/10.14283/jpad.2023.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.3