Abstract
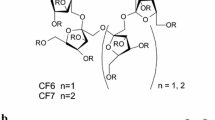
Direct liquid chromatographic methods were developed to investigate the enantioseparation of 19 β-lactams on three cyclodextrin-bonded chiral stationary phases: permethyl-β-cyclodextrin, β-cyclodextrin and R,S-hydroxypropyl-β-cyclodextrin, prepared by a novel synthetic route. 17 of the 19 β-lactam stereoisomers were partially or baseline-separated on at least one of the tested chiral stationary phases. The influence of the structures of the β-lactams (the positions and types of the substituents, the size of the attached rings) on the enantiomer separation is discussed. The permethylated β-cyclodextrin selector proved to be the most effective one for the tested analytes.

Similar content being viewed by others
References
Matagne A, Lamotte-Brasseur J, Freré JM (1998) J Biochem 330:581–598
Matagne A, Dubus A, Galleni M, Freré JM (1999) Nat Prod Rep 16:1–19
Lamotte J, Dive G, Ghuysen JM (1991) Eur J Med Chem 26:43–50
Freré JM (1995) Microbiol 16:385–395
Palomo C, Aizpurua JM, Ganboa I, Oiarbide M (2001) Synlett 12:1813–1826
Palomo C, Ganboa I, Oiarbide M, Sciano GT, Miranda JL (2002) Arkivoc 5:8–16
Wasserman HH, Matsuyama H, Robinson RP (2002) Tetrahedron 58:7177–7190
Patel RN, Howell J, Chidambaram R, Benoit S, Kant J (2003) Tetrahedron Asymmetr 14:3673–3677
Forró E, Fülöp F (2004) Mini Rev Org Chem 93-102
Fülöp F (2001) Chem Rev 101:2181–2204
Pirkle WH, Spence PL (1998) Chirality 10:430–433
Pirkle WH, Finn JM, Schreiner JL (1981) J Amer Chem Soc 103:3964–3966
Ficarra R, Calabro ML, Tommasini S, Constatino D (1996) Chromatographia 43:365–378
Pirkle WH, Tsipouras A, Hyun MH (1986) J Chromatog 43:365–378
Cirilli R, DelGiudice MR, Ferretti R, La Torre F (2001) J Chromatog A 923:27–36
Péter A, Árki A, Forró E, Fülöp F, Armstrong DW (2005) Chirality 17:193–200
Lee CS, Chen HH (1994) J Chin Chem Soc 41:187–190
Okamato Y, Senoh T, Nakane H, Hatada K (1989) Chirality 1:216–222
Berkecz R, Török R, Ilisz I, Forró E, Fülöp F, Armstrong DW, Péter A (2006) Chromatographia 63:537–543
Ficarra R, Calabro ML, Alcaro S, Tomassini S, Melardi S, Ficarra P (2000) Chromatographia 51:411–416
Sun P, Wang C, Péter A, Forró E, Armstrong DW (2006) J Liq Chromatogr Rel Technol 29:1847–1860
Varga G, Tárkányi G, Németh K, Iványi R, Jicsinszky L, Tőke O, Visy J, Szente L, Szemán J, Simonyi M (2010) J Pharm Biomed Anal 51:84–89
Connors AK (1995) J Pharm Sci 84:843–848
Acknowledgments
The authors are grateful to Ms. Zs. Zachár and Ms. O. Nemes for their valuable technical assistance. This work was supported financially by the grants Jedlik Ányos 00180/2007, NKFP_07_A3_NATURSEP and OTKA 67563.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at: 8th Balaton Symposium on High-Performance Separation Methods, Siofok, Hungary, September 2–4, 2009.
Rights and permissions
About this article
Cite this article
Fodor, G., Ilisz, I., Szemán, J. et al. LC Enantioseparation of β-Lactam Stereoisomers through the Use of β-Cyclodextrin-Based Chiral Stationary Phases. Chroma 71 (Suppl 1), 29–34 (2010). https://doi.org/10.1365/s10337-010-1514-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-010-1514-0