Abstract
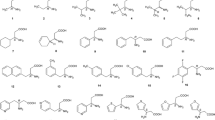
A novel series of nine chiral analogs of spirobrassinin, which have potential biological activity, was separated for the first time on three different derivatized cyclofructan chiral stationary phases in the normal phase mode. The effects of mobile phase composition, the type and concentration of polar modifier, additives, and the analyte structure on the retention and enantioseparation were studied. The results proved that for cyclofructan-based chiral stationary phases, the R-naphthylethyl carbamate cyclofructan 6 provides the best separation efficiency for the analyzed compounds. The effect of temperature on the separation was also investigated and the corresponding thermodynamic parameters were evaluated from linear Van’t Hoff plots (lnk or lnα versus 1/T). It was found that the enantioseparation was enthalpy controlled. In addition, the elution order of the enantiomers was determined in all the cases.






Similar content being viewed by others
References
Pedras MSC, Yaya EE, Glawischnig E (2011) The phytoalexins from cultivated and wild crucifers: chemistry and biology. Nat Prod Rep 28:1381–1405
Monde K, Taniguchi T, Miura N, Kutschy P, Čurillová Z, Pilátová M, Mojžiš J (2005) Chiral cruciferous phytoalexins: preparation, absolute configuration, and biological activity. Bioorg Med Chem 13:5206–5212
Moody CJ, Roffey JRA, Stephens MA, Stratford IJ (1997) Synthesis and cytotoxic activity of indolyl thiazoles. Anticancer Drugs 8:489–499
Kutschy P, Salayová A, Čurillová Z, Kožár T, Mezencev R, Mojžiš J, Pilátová M, Balentová E, Pazdera P, Sabol M, Zburová M (2009) 2-(Substituted phenyl) amino analogs of 1-methoxyspirobrassinol methyl ether: synthesis and anticancer activity. Bioorg Med Chem 17:3698–3712
Pilátová M, Šarišský M, Kutschy P, Miroššay A, Mezencev R, Čurillová Z, Suchý M, Monde K, Mirossay L, Mojžiš J (2005) Cruciferous phytoalexins: antiproliferative effects in T-Jurkat leukemics cells. Leukemia Res 29:415–421
Mezencev R, Mojžiš R, Pilátová M, Kutschy P (2003) Antiproliferative and cancer chemopreventive activity of phytoalexins: focus on indole phytoalexins from crucifers. Neoplasma 50:239–245
Monde K, Harada N, Takasugi M, Kutschy P, Suchý M, Dzurilla M (2000) Enantiomeric excess of a cruciferous phytoalexin, spirobrassinin, and its enantiomeric enrichment in an achiral HPLC system. J Nat Prod 63:1312–1314
Gondová T, Petrovaj J, Kutschy P, Čurillová Z, Salayová A, Fabián M, Armstrong DW (2011) Enantioseparation of novel amino analogs of indole phytoalexins on macrocyclic glycopeptide-based chiral stationary phase. Chromatographia 74:751–757
Gondová T, Petrovaj J, Kutschy P, Armstrong DW (2013) Stereoselective separation of spiroindoline phytoalexins on R-naphthylethyl cyclofructan 6-based chiral stationary phase. J Chromatogr A 1272:100–105
Sun P, Wang C, Breitbach ZS, Zhang Y, Armstrong DW (2009) Development of new HPLC chiral stationary phases based on native and derivatized cyclofructans. Anal Chem 81:10215–10226
Berthod A (2010) Chiral recognition in separation methods. Springer, Heidelberg
Kalíková K, Janečková L, Armstrong DW, Tesařová E (2011) Characterization of new R-naphthylethyl cyclofructan 6 chiral stationary phase and its comparison with R-naphthyl-ethyl beta-cyclodextrin-based column. J Chromatogr A 1218:1393–1398
Aranyi A, Ilisz I, Pataj Z, Szatmári I, Fülöp F, Armstrong DW, Péter A (2011) High- performance liquid chromatographic enantioseparation of Betti base analogs on a newly developed isopropyl carbamate-cyclofructan 6-based chiral stationary phase. Chirality 23:549–556
Frink LA, Khan MA, Kürti L, Falck JR, Paudyal MP, Jat JL, Armstrong DW (2014) Enantiomeric separations of N-H/N-Me aziridines utilizing GC and HPLC. Chromatographia 77:1607–1612
Hroboňová K, Moravčík J, Lehotay J, Armstrong DW (2015) Determination of methionine enantiomers by HPLC on the cyclofructan chiral stationary phase. Anal. Methods 7:4577–4582
Stavrou IJ, Breitbach YS, Christodoulou CPK (2015) Combined use of cyclofructans and an amino acid ester-based ionic liquid for the enantioseparation of huperzine A and coumarin derivatives in CE. Electrophoresis 36:3061–3068
Geryk R, Vozka J, Kalíková K, Tesařová E (2013) HPLC method for chiral separation and quantification of antidepressant citalopram and its precursor citadiol. Chromatographia 76:483–489
Breitbach AS, Lim Y, Xu QL, Kürti L, Armstrong DW, Breitbach ZS (2016) Enantiomeric separations of α-aryl ketones with cyclofructan chiral stationary phases via high performance liquid chromatography and supercritical fluid chromatography. J Chromatogr A 1427:45–54
Budovská M, Bago Pilátová M, Mojžiš J (2014) 2´-Amino analogs of spirooxindole cruciferous phytoalexin spirobrassinin: Synthesis and antiproliferative activity. Presented in part at XXXI. Conference of Organic Chemists, Smolenice, Slovakia
Vozka J, Kalíková K, Roussel C, Armstrong DW, Tesařová E (2013) An insight into the use of dimethylphenyl carbamate cyclofructan 7 chiral stationary phase in supercritical fluid chromatography: the basic comparison with HPLC. J Sep Sci 36:1711–1719
Chankvetadze B (2002) Enantiomer migration order in chiral capillary electrophoresis. Electrophoresis 23:4022–4035
Feibush B, Gil-Av E (1970) Interaction between assymetric solutes and solvents: peptide derivatives as stationary phases in gas liquid partition chromatography. Tetrahedron 26:136–1368
Špánik I, Krupčík J, Schurig V (1999) Comparison of two methods for the gas chromatographic determination of thermodynamic parameters of enantioselectivity. J Chromatogr A 843:123–128
Péter A, Vékes E, Armstrong DW (2002) Effects of temperature on retention of chiral compounds on a ristocetin A chiral stationary phase. J Chromatogr A 958:89–107
Rojkovičová T, Lehotay J, Krupčík J, Fedurcová A, Čižmárik J, Armstrong DW (2004) Study of the mechanism of enantioseparation. VII. Effect of temperature on retention of some enantiomers of phenylcarbamic acid derivates on a teicoplanin aglycone chiral stationary phase. J Liq Chromatogr Rel Technol 27:1653–1670
Lämmerhofer M (2010) Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J Chromatogr A 1217:814–856
Oberleitner WR, Maier NM, Lindner W (2002) Enantioseparation of various amino acid derivatives on a quinine based chiral anion-exchange selector at variable temperature conditions. Influence of structural parameters of the analytes on the apparent retention and enantioseparation characteristics. J Chromatogr A 960:97–108
Sipos L, Ilisz I, Pataj Z, Szakonyi Z, Fülöp F, Armstrong DW, Péter A (2010) High- performance liquid chromatographic enantioseparation of monoterpene-based 2-amino carboxylic acids on macrocyclic glycopeptide based-phases. J Chromatogr A 1217:6956–6963
Acknowledgements
The authors gratefully acknowledge the financial support received from the Scientific Grant Agency VEGA of Slovak Republic (Grant No. 1/0253/16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Moskaľová, M., Kozlov, O., Gondová, T. et al. HPLC Enantioseparation of Novel Spirobrassinin Analogs on the Cyclofructan Chiral Stationary Phases. Chromatographia 80, 53–62 (2017). https://doi.org/10.1007/s10337-016-3212-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3212-z