Abstract
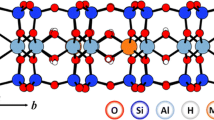
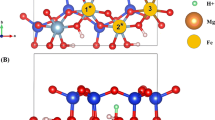
Sorption of U(VI) on clay and related minerals has been inspected experimentally and computationally because of its central role in safety considerations of geological repositories for highly radioactive waste. Np(V), which also has long half-life isotopes, has received considerably less attention. The purpose of the present study was to investigate computationally the adsorption of Np(V) on a clay-mineral surface and to compare it to adsorption of U(VI). As a sample case study, adsorption of Np(V) at the (110) edge surface of the common clay mineral montmorillonite was modeled. The density functional approach and periodic supercell models were applied. Mono- and bidentate adsorption complexes with coordination numbers 4 and 5 were inspected and compared to corresponding U(VI) species. While U(VI) prefers bidentate adsorption complexes with varying coordination numbers, Np(V) is more stable when monodentate-coordinated with a coordination number of four. In line with its smaller hydrolysis constant in aqueous solution, Np(V) shows a lower tendency to form monohydroxides on the mineral surface compared to U(VI). As no experimental geometry parameters are available for Np(V) adsorbed on montmorillonite, the results were compared tentatively to EXAFS data for adsorption at kaolinite and good agreement for the geometry changes due to adsorption was found for the more preferred adsorbed species.
Similar content being viewed by others
References
Allen, P.G., Bucher, J.J., Shuh, D.K., Edelstein, N.M., and Reich, T. (1997) Investigation of aquo and chloro complexes of UO 2+2 , NpO +2 , Np4+, and Pu3+ by X-ray absorption fine structure spectroscopy. Inorganic Chemistry, 36, 4676–4683.
Amayri, S., Banik, N.L., Breckheimer, M., Buda, R.A., Burger, S., Drebert, J., Jermolajev, A., Kratz, J.V., Kuczewski, B., Kutscher, D., Reich, T.Y., Reich, T., and Trautmann, N. (2008) Interaction of neptunium and plutonium with humic substances and kaolinite. Pp. 141–216 in: Migration of Actinides in the System Clay, Humic Substances, Aquifer (C.M. Marquardt, editor). FZKA 7407, Forschungszentrum Karlsruhe, Karlsruhe, Germany.
Amayri, S., Jermolajev, A., and Reich, T. (2011) Neptunium(V) sorption on kaolinite. Radiochimica Acta, 99, 349–357.
Antonio, M.R., Soderholm, L., Williams, C.W., Blaudeau, J.P., and Bursten, B.E. (2001) Neptunium redox speciation. Radiochimica Acta, 89, 17–25.
Arai, Y., Moran, P.B., Honeyman, B.D., and Davis, J.A. (2007) In situ spectroscopic evidence for neptunium(V)-carbonate inner-sphere and outer-sphere ternary surface complexes on hematite surfaces. Environmental Science & Technology, 41, 3940–3944.
Arnold, T., Scheinost, A.C., Baumann, N., and Brendler, V. (2006) Surface speciation of uranyl (VI) on gibbsite: A combined spectroscopic approach. P. 53 in: Annual Report (G. Bernhard, editor). Institute of Radiochemistry, Dresden-Rossendorf, Germany.
Benedicto, A., Begg, J.D., Zhao, P., Kersting, A.B., Missana, T., and Zavarin, M. (2014). Effect of major cation water composition on the ion exchange of Np(V) on montmorillonite: NpO +2 -Na+-K+-Ca2+-Mg2+ selectivity coefficients. Applied Geochemistry, 47, 177–185.
Bidoglio, G., Tanet, G., and Chatt, A. (1985) Studies on neptunium (V) carbonate complexes under geologic repository conditions. Radiochimica Acta, 38, 21–26.
Blöchl, P.E. (1994) Projector augmented wave method. Physical Review B, 50, 17953–17979.
Bradbury, M.H. and Baeyens, B. (2005a) Experimental measurements and modeling of sorption competition on montmorillonite. Geochimica et Cosmochimica Acta, 69, 4187–4197.
Bradbury, M.H. and Baeyens, B. (2005b) Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: Linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochimica et Cosmochimica Acta, 69, 875–892.
Butler, D. (2010) France digs deep for nuclear waste. Nature, 466, 804–805.
Catalano, J.G. and Brown, G.E. (2005) Uranyl adsorption onto montmorillonite: Evaluation of binding sites and carbonate complexation. Geochimica et Cosmochimica Acta, 69, 2995–3005.
Chisholm-Brause, C., Conradson, S.D., Buscher, C.T., Eller, P.G., and Morris, D.E. (1994) Speciation of uranyl sorbed at multiple binding-sites on montmorillonite. Geochimica et Cosmochimica Acta, 58, 3625–3631.
Churakov, S.V. (2007) Structure and dynamics of the water films confined between edges of pyrophyllite: A first principle study. Geochimica et Cosmochimica Acta, 71, 1130–1144.
Combes, J.M., Chisholm-Brause, C.J., Brown, G.E., Parks, G.A., Conradson, S.D., Eller, P.G., Triay, I.R., Hobart, D.E., and Meijer, A. (1992) EXAFS spectroscopic study of neptunium(V) sorption at the alpha-FeOOH water interface. Environmental Science & Technology, 26, 376–382.
Cotton, S. (2006) Lanthanide and Actinide Chemistry. Wiley, Chichester, UK.
Del Nero, M., Assada, A., Madé, B., Barillon, R., and Duplâtre, G. (2004) Surface charges and Np(V) sorption on amorphous Al and Fe silicates. Chemical Geology, 211, 15–45.
Den Auwer, C., Drot, R., Simoni, E., Conradson, S.D., Gailhanou, M., and de Leon, J.M. (2003) Grazing incidence XAFS spectroscopy of uranyl sorbed onto TiO2 rutile surfaces. New Journal of Chemistry, 27, 648–655.
Denecke, M.A., Reich, T., Pompe, S., Bubner, M., Heise, K.H., Nitsche, H., Allen, P.G., Bucher, J.J., Edelstein, N.M., and Shuh, D.K. (1997) Differentiating between monodentate and bidentate carboxylate ligands coordinated to uranyl ions using EXAFS. Journal de Physique IV, 7, 637–638.
Dent, A.J., Ramsay, J.D.F., and Swanton, S.W. (1992) An EXAFS study of uranyl-ion in solution and sorbed onto silica and montmorillonite clay colloids. Journal of Colloid and Interface Science, 150, 45–60.
Fröhlich, D.R. (2015). Sorption of neptunium on clays and clay minerals — a review. Clays and Clay Minerals, 63, 262–276.
Geckeis, H., Lützenkirchen, J., Polly, R., Rabung, T., and Schmidt, M. (2013) Mineral-water interface reactions of actinides. Chemical Reviews, 113, 1016–1062.
Greathouse, J.A. and Cygan, R.T. (2005) Molecular dynamics simulation of uranyl(VI) adsorption equilibria onto an external montmorillonite surface. Physical Chemistry Chemical Physics, 7, 3580–3586.
Greathouse, J.A. and Cygan, R.T. (2006) Water structure and aqueous uranyl(VI) adsorption equilibria onto external surfaces of beidellite, montmorillonite, and pyrophyllite: Results from molecular simulations. Environmental Science & Technology, 40, 3865–3871.
Grenthe, I., Fuger, J., Konings, R., Lemire, R., Muller, A., Nguyen-Trung, C., and Wanner, H. (2004) Chemical Thermodynamics of Uranium. OECD Publications, Paris.
Gückel, K., Rossberg, A., Müller, K., Brendler, V., Bernhard, G., and Foerstendorf, H. (2013) Spectroscopic identification of binary and ternary surface complexes of Np(V) on gibbsite. Environmental Science & Technology, 47, 14418–14425.
Hennig, C., Reich, T., Dähn, R., and Scheidegger, A.M. (2002) Structure of uranium sorption complexes at montmorillonite edge sites. Radiochimica Acta, 90, 653–657.
Ikeda-Ohno, A., Hennig, C., Rossberg, A., Funke, H., Scheinost, A.C., Bernhard, G., and Yaita, T. (2008) Electrochemical and complexation behavior of neptunium in aqueous perchlorate and nitrate solutions. Inorganic Chemistry, 47, 8294–8305.
Kosmulski, M. (2012) IEP as a parameter characterizing the pH-dependent surface charging of materials other than metal oxides. Advances in Colloid and Interface Science, 171-172, 77–86.
Kowal-Fouchard, A., Drot, R., Simoni, E., and Ehrhardt, J.J. (2004) Use of spectroscopic techniques for uranium (VI)/ montmorillonite interaction modeling. Environmental Science & Technology, 38, 1399–1407.
Kremleva, A., Krüger, S., and Rösch, N. (2008) Density functional model studies of uranyl adsorption on (001) surfaces of kaolinite. Langmuir, 24, 9515–9524.
Kremleva, A., Krüger, S., and Rösch, N. (2011) Uranyl adsorption at (010) edge surfaces of kaolinite: A density functional study. Geochimica et Cosmochimica Acta, 75, 706–718.
Kremleva, A., Krüger, S., and Rösch, N. (2015) Uranyl adsorption at solvated edge surfaces of 2:1 smectites. A density functional study. Physical Chemistry Chemical Physics, 17, 13757–13768.
Kremleva, A., Krüger, S., and Rösch, N. (2016) Toward a reliable energetics of adsorption at solvated mineral surfaces: A computational study of uranyl (VI) on 2:1 clay minerals. Journal of Physical Chemistry C, 120, 324–335.
Křepelová, A., Reich, T., Sachs, S., Drebert, J., and Bernhard, G. (2008) Structural characterization of U(VI) surface complexes on kaolinite in the presence of humic acid using EXAFS spectroscopy. Journal of Colloid and Interface Science, 319, 40–47.
Kresse, G. and Furthmüller, J. (1996a) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 6, 15–50.
Kresse, G. and Furthmüller, J. (1996b) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B, 54, 11169–11186.
Kresse, G. and Hafner, J. (1993a) Ab initio molecular dynamics for liquid metals. Physical Review B, 47, 558–561.
Kresse, G. and Hafner, J. (1993b) Ab initio molecular dynamics for open shell transition metals. Physical Review B, 48, 13115–13118.
Kresse, G. and Hafner, J. (1994) Ab initio molecular dynamics simulation of the liquid metal amorphous semiconductor transition in germanium. Physical Review B, 49, 14251–14269.
Kresse, G. and Joubert, D. (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 59, 1758–1775.
Lectez, S., Roques, J., Salanne, M., and Simoni, E. (2012) Car-Parrinello molecular dynamics study of the uranyl behaviour at the gibbsite/water interface. The Journal of Chemical Physics, 137, 154705.
Lemire, R., Fuger, J., Nitsche, H., Potter, P., Rand, M., Rydberg, J., Spahiu, K., Sullivan, J., Ullman, W., Vitorge, P., and Wanner, H. (2001) Chemical Thermodynamics of Neptunium and Plutonium. Elsevier, Amsterdam.
Liu, X., Lu, X., Sprik, M., Cheng, J., Meijer, E. J., and Wang, R. (2013) Acidity of edge surface sites of montmorillonite and kaolinite. Geochimica et Cosmochimica Acta, 117, 180–190.
Liu, X.D., Cheng, J., Sprik, M., Lu, X.C., and Wang, R.C. (2014) Surface acidity of 2:1-type dioctahedral clay minerals from first principles molecular dynamics simulations. Geochimica et Cosmochimica Acta, 140, 410–417.
Lo, C. and Trout, B.L. (2004). Density-functional theory characterization of acid sites in chabazite. Journal of Catalysis, 227, 77–89.
MacDonald, A.H., Pickett, W.E., and Koelling, D.D. (1980) A linearized relativistic augmented-plane-wave method utilizing approximate pure spin basis functions. Journal of Physics C — Solid State Physics, 13, 2675–2683.
MacDonald, A.H. and Vosko, S.H. (1979) Relativistic density functional formalism. Journal of Physics C — Solid State Physics, 12, 2977–2990.
Marques Fernandes, M., Baeyens, B., Dähn, R., Scheinost, A.C., and Bradbury, M.H. (2012) U(VI) sorption on montmorillonite in the absence and presence of carbonate: A macroscopic and microscopic study. Geochimica et Cosmochimica Acta, 93, 262–277.
Morris, D.E., Chisholm-Brause, C.J., Barr, M.E., Conradson, S.D., and Eller, P.G. (1994) Optical spectroscopic studies of the sorption of UO 2+2 species on a reference smectite. Geochimica et Cosmochimica Acta, 58, 3613–3623.
Müller, K., Foerstendorf, H., Brendler, V., and Bernhard, G. (2009) Sorption of Np(V) onto TiO2, SiO2, and ZnO: An in situ ATR FT-IR spectroscopic study. Environmental Science & Technology, 43, 7665–7670.
Müller, K., Foerstendorf, H., Meusel, T., Brendler, V., Lefevre, G., Comarmond, M.J., and Payne, T.E. (2012) Sorption of U(VI) at the TiO2-water interface: An in situ vibrational spectroscopic study. Geochimica et Cosmochimica Acta, 76, 191–205.
Niitsu, Y., Sato, S., Ohashi, H., Sakamoto, Y., Nagao, S., Ohnuki, T., and Muraoka, S. (1997) Effects of humic acid on the sorption of neptunium(V) on kaolinite. Journal of Nuclear Materials, 248, 328–332.
Pauling, L. (1929) The principles determining the structure of complex ionic crystals. Journal of the American Chemical Society, 51, 1010–1026.
Perdew, J.P. and Wang, Y. (1992) Accurate and simple analytic representation of the electron gas correlation energy. Physical Review B, 45, 13244–13249.
Reich, T., Bernhard, G., Geipel, G., Funke, H., Hennig, C., Rossberg, A., Matz, W., Schell, N., and Nitsche, H. (2000) The Rossendorf beam line ROBL — a dedicated experimental station for XAFS measurements of actinides and other radionuclides. Radiochimica Acta, 88, 633–637.
Reich, T., Reich, T.Y., Amayri, S., Drebert, J., Banik, N.L., Buda, R.A., Kratz, J.V., and Trautmann, N. (2007) Application of XAFS spectroscopy to actinide environmental science. AIP Conference Proceedings, 882, 179–183.
Schlegel, M.L. and Descostes, M. (2009) Uranium uptake by hectorite and montmorillonite: A solution chemistry and polarized EXAFS study. Environmental Science & Technology, 43, 8593–8598.
Schmeide, K. and Bernhard, G. (2010) Sorption of Np(V) and Np(IV) onto kaolinite: Effects of pH, ionic strength, carbonate and humic acid. Applied Geochemistry, 25, 1238–1247.
Sylwester, E.R., Hudson, E.A., and Allen, P.G. (2000) The structure of uranium (VI) sorption complexes on silica, alumina, and montmorillonite. Geochimica et Cosmochimica Acta, 64, 2431–2438.
Tazi, S., Rotenberg, B., Salanne, M., Sprik, M., and Sulpizi, M. (2012) Absolute acidity of clay edge sites from ab-initio simulations. Geochimica et Cosmochimica Acta, 94, 1–11.
Tettenhorst, R. and Roberson, H.E. (1973) X-ray-diffraction aspects of montmorillonites. American Mineralogist, 58, 73–80.
Tochiyama, O., Yamazaki, H., and Mikami, T. (1996) Sorption of neptunium(V) on various aluminum oxides and hydrous aluminum oxides. Radiochimica Acta, 73, 191–198.
Turner, D.R., Pabalan, R.T., and Bertetti, F.P. (1998) Neptunium(V) sorption on montmorillonite: An experimental and surface complexation modeling study. Clays and Clay Minerals, 46, 256–269.
Vallet, V., Moll, H., Wahlgren, U., Szabo, Z., and Grenthe, I. ( 2003) Structure and bonding in solution of dioxouranium(VI) oxalate complexes: Isomers and intramolecular ligand exchange. Inorganic Chemistry, 42, 1982–1993.
Wahlgren, U., Moll, H., Grenthe, I., Schimmelpfennig, B., Maron, L., Vallet, V., and Gropen, O. (1999) Structure of uranium(VI) in strong alkaline solutions. A combined theoretical and experimental investigation. Journal of Physical Chemistry A, 103, 8257–8264.
Zavarin, M., Powell, B.A., Bourbin, M., Zhao, P., and Kersting, A.B. (2012) Np(V) and Pu(V) ion exchange and surface-mediated reduction mechanisms on montmorillonite. Environmental Science & Technology, 46, 2692–2698.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kremleva, A., Krüger, S. Comparative Computational Study of NP(V) and U(VI) Adsorption on (110) Edge Surfaces of Montmorillonite. Clays Clay Miner. 64, 438–451 (2016). https://doi.org/10.1346/CCMN.2016.0640408
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2016.0640408