Abstract
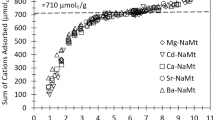
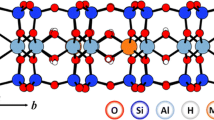
Although multiple types of adsorption sites have long been observed in montmorillonite, a consistent explanation about the chemical structure of these adsorption sites has not yet been established. Identifying the cation interlayer adsorption sites based on the octahedral cation distribution on montmorillonite was investigated in this study by using a Density Functional Theory (DFT) simulation. A clay structural model (H[Al6MgFe]Si16O40(OH)8) with a similar composition to Wyoming SWy-1 montmorillonite was built, where two octahedral Al were respectively substituted by Fe and Mg, and H+ was the charge compensating cation. This model had twenty-one different possible configurations as a function of the distribution of octahedral Al, Fe, and Mg cations. The DFT simulations of 15 of these different configurations showed no preference for the formation of any configuration with a specific octahedral Fe-Mg distance. However, the H+ adsorption energy was separated into three distinct groups based on the number of octahedral jumps from Fe to Mg atoms. The H+ adsorption energy significantly decreased with increasing number of octahedral jumps from Fe to Mg. Assuming an even probability of occurrence of 21 octahedral structures in montmorillonite, the percentages of these three groups are 43, 43, and 14%, respectively, which are very close to the three major sites on montmorillonite from published cation adsorption data. These DFT simulations offer an entirely new explanation for the location and chemical structure of the three major adsorption sites on montmorillonite, namely, all three sites are in the interlayer, and their adsorption strengths are a function of the number of octahedral jumps from Fe to Mg atoms.



Similar content being viewed by others
Change history
20 February 2020
This article was updated to correct changes to the text made during production. The phrase “Fe can be placed in one of three ways at <Emphasis Type="Italic">j</Emphasis> = 1 and 2” was updated.
References
Agmon, N. (1999). Proton solvation and proton mobility. Israel Journal of Chemistry, 39, 493–502.
Benson, L. V. (1982). A tabulation and evaluation of ion exchange data on smectites. Environmental Geology, 4, 23–29.
Blöchl, P. E. (1994). Projector augmented-wave method. Physical review B, 50, 17953–17979.
Bradbury, M. H., & Baeyens, B. (1997). A mechanistic description of Ni and Zn sorption on Na-montmorillonite. Part II: Modelling. Journal of Contaminant Hydrology, 27, 223–248.
Carey, F.A., & Sundberg, R.J. (2007). Advanced organic chemistry. Part A: Structure and mechanisms. Springer Science & Business Media.
Chatterjee, A., Iwasaki, T., Ebina, T., & Miyamoto, A. (1999). A DFT study on clay-cation-water interaction in montmorillonite and beidellite. Computational Materials Science, 14, 119–124.
Cornell, R. (1993). Adsorption of cesium on minerals: A review. Journal of Radioanalytical and Nuclear Chemistry, 171, 483–500.
Cuadros, J., Sainz-Diaz, C. I., Ramirez, R., & Hernandez-Laguna, A. (1999). Analysis of Fe segregation in the octahedral sheet of bentonitic illite-smectite by means of FTIR, 27Al MAS NMR and reverse Monte Carlo simulations. American Journal of Science, 299, 289–308.
Drits, V. A., McCarty, D. K., & Zviagina, B. B. (2006). Crystal-chemical factors responsible for the distribution of octahedral cations over trans- and cis-sites in dioctahedral 2:1 layer silicates. Clays and Clay Minerals, 54, 131–152.
Dzene, L., Tertre, E., Hubert, F., & Ferrage, E. (2015). Nature of the sites involved in the process of cesium desorption from vermiculite. Journal of Colloid and Interface Science, 455, 254–260.
Emmerich, K., & Kahr, G. (2001). The cis-and trans-vacant variety of a montmorillonite: an attempt to create a model smectite. Applied Clay Science, 20, 119–127.
Escamilla-Roa, E., Nieto, F., & Sainz-Díaz, C. I. (2016). Stability of the hydronium cation in the structure of illite. Clays and Clay Minerals, 64, 413–424.
Ferreira, D. R., Schulthess, C. P., & Giotto, M. V. (2011). An investigation of strong sodium retention mechanisms in nanopore environments using nuclear magnetic resonance spectroscopy. Environmental Science and Technology, 46, 300–306.
Finck, N., Schlegel, M. L., & Bauer, A. (2015). Structural iron in dioctahedral and trioctahedral smectites: A polarized XAS study. Physics and Chemistry of Minerals, 42, 847–859.
Gajdoš, M., Hummer, K., Kresse, G., Furthmüller, J., & Bechstedt, F. (2006). Linear optical properties in the projector-augmented wave methodology. Physical Review B, 73, (045112), 1–9.
Hernández-Laguna, A., Escamilla-Roa, E., Timón, V., Dove, M. T., & Sainz-Díaz, C. I. (2006). DFT study of the cation arrangements in the octahedral and tetrahedral sheets of dioctahedral 2:1 phyllosilicates. Physics and Chemistry of Minerals, 33, 655–666.
Hernández-Haro, N., Ortega-Castro, J., Pruneda, M., Sainz-Díaz, C. I., & Hernández-Laguna, A. (2014). Theoretical study on the influence of the Mg2+ and Al3+ octahedral cations on the vibrational spectra of the hydroxy groups of dioctahedral 2:1 phyllosilicate models. Journal of Molecular Modeling, 20, (2402), 1–10.
Jacquier, P., Ly, J., & Beaucaire, C. (2004). The ion-exchange properties of the Tournemire argillite: I. Study of the H, Na, K, Cs, Ca and Mg behaviour. Applied Clay Science, 26, 163–170.
Kaufhold, S., Kremleva, A., Krüger, S., Rösch, N., Emmerich, K., & Dohrmann, R. (2017). Crystal-chemical composition of dicoctahedral smectites: An energy-based assessment of empirical relations. ACS Earth and Space Chemistry, 1, 629–636.
Kresse, G., & Furthmüller, J. (1996). Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B, 54, 11169–11186.
Kresse, G., & Joubert, D. (1999). From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 59, 1758–1775.
Lantenois, S., Muller, F., Bény, J. M., Mahiaoui, J., & Champallier, R. (2008). Hydrothermal synthesis of beidellites: Characterization and study of the cis-and trans-vacant character. Clays and Clay Minerals, 56, 39–48.
Lavikainen, L. P., Tanskanen, J. T., Schatz, T., Kasa, S., & Pakkanen, T. A. (2015). Montmorillonite interlayer surface chemistry: Effect of magnesium ion substitution on cation adsorption. Theoretical Chemistry Accounts, 134, (51), 1–7.
Macht, F., Eusterhues, K., Pronk, G. J., & Totsche, K. U. (2011). Specific surface area of clay minerals: Comparison between atomic force microscopy measurements and bulk-gas (N2) and-liquid (EGME) adsorption methods. Applied Clay Science, 53, 20–26.
Martin, L. A., Wissocq, A., Benedetti, M. F., & Latrille, C. (2018). Thallium (Tl) sorption onto illite and smectite: Implications for Tl mobility in the environment. Geochimica et Cosmochimica Acta, 230, 1–16.
McKinley, J. P., Zachara, J. M., Smith, S. C., & Turner, G. D. (1995). The influence of uranyl hydrolysis and multiple site-binding reactions on adsorption of U(VI) to montmorillonite. Clays and Clay Minerals, 43, 586–598.
Missana, T., Benedicto, A., García-Gutiérrez, M., & Alonso, U. (2014). Modeling cesium retention onto Na-, K- and Ca-smectite: Effects of ionic strength, exchange and competing cations on the determination of selectivity coefficients. Geochimica et Cosmochimica Acta, 128, 266–277.
Monkhorst, H. J., & Pack, J. D. (1976). Special points for Brillouin-zone integrations. Physical Review B, 13, 5188–5192.
Motellier, S., Ly, J., Gorgeon, L., Charles, Y., Hainos, D., Meier, P., & Page, J. (2003). Modelling of the ion-exchange properties and indirect determination of the interstitial water composition of an argillaceous rock. Application to the Callovo-Oxfordian low-water-content formation. Applied Geochemistry, 18, 1517–1530.
Muller, F., Besson, G., Manceau, A., & Drits, V. A. (1997). Distribution of isomorphous cations within octahedral sheets in montmorillonite from Camp-Bertaux. Physics and Chemistry of Minerals, 24, 159–166.
Muller, F., Drits, V., Plançon, A., & Robert, J. L. (2000). Structural transformation of 2:1 dioctahedral layer silicates during dehydroxylation-rehydroxylation reactions. Clays and Clay Minerals, 48, 572–585.
Neumann, A., Petit, S., & Hofstetter, T. B. (2011). Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy. Geochimica et Cosmochimica Acta, 75, 2336–2355.
Nolin, D. (1997). Rétention de radioéléments à vie longue par des matériaux argileux. Influence d’anions contenus dans les eaux naturelles. Ph.D. Thesis, Universite Pierre Et Marie Curie, Paris 6.
Norrish, K. (1954). The swelling of montmorillonite. Discussions of the Faraday Society, 18, 120–134.
Ohkubo, T., Okamoto, T., Kawamura, K., Guégan, R., Deguchi, K., Ohki, S., Shimizu, T., Tachi, Y., & Iwadate, Y. (2018). New insights into the Cs adsorption on montmorillonite clay from 133Cs solid-state NMR and density functional theory calculations. The Journal of Physical Chemistry A, 122, 9326–9337.
Ortega-Castro, J., Hernández-Haro, N., Dove, M. T., Hernández-Laguna, A., & Sainz-Díaz, C. I. (2010). Density functional theory and Monte Carlo study of octahedral cation ordering of Al/Fe/Mg cations in dioctahedral 2:1 phyllosilicates. American Mineralogist, 95, 209–220.
Pauling, L. (1960). The Nature of the chemical bond. Ithaca, NY: Cornell University Press.
Perdew, J. P., Burke, K., & Ernzerhof, M. (1996). Generalized gradient approximation made simple. Physical Review Letters, 77, 3865–3868.
Poinssot, C., Baeyens, B., & Bradbury, M. H. (1999). Experimental and modelling studies of caesium sorption on illite. Geochimica et Cosmochimica Acta, 63, 3217–3227.
Robin, V., Tertre, E., Beaufort, D., Regnault, O., Sardini, P., & Descostes, M. (2015). Ion exchange reactions of major inorganic cations (H+, Na+, Ca2+, Mg2+ and K+) on beidellite: Experimental results and new thermodynamic database. Toward a better prediction of contaminant mobility in natural environments. Applied Geochemistry, 59, 74–84.
Robin, V., Tertre, E., Beaucaire, C., Regnault, O., & Descostes, M. (2017). Experimental data and assessment of predictive modeling for radium ion-exchange on beidellite, a swelling clay mineral with a tetrahedral charge. Applied Geochemistry, 85, 1–9.
Rotenberg, B., Morel, J. P., Marry, V., Turq, P., & Morel-Desrosiers, N. (2009). On the driving force of cation exchange in clays: Insights from combined microcalorimetry experiments and molecular simulation. Geochimica et Cosmochimica Acta, 73, 4034–4044.
Sainz-Diaz, C. I., Hernández-Laguna, A., & Dove, M. T. (2001). Theoretical modelling of cis-vacant and trans-vacant configurations in the octahedral sheet of illites and smectites. Physics and Chemistry of Minerals, 28, 322–331.
Sawhney, B. (1972). Selective sorption and fixation of cations by clay minerals: A review. Clays and Clay Minerals, 20, 93–100.
Schulthess, C. P., & Huang, C. P. (1990). Adsorption of heavy metals by silicon and aluminum oxide surfaces on clay minerals. Soil Science Society of America Journal, 54, 679–688.
Schulthess, C. P., Taylor, R. W., & Ferreira, D. R. (2011). The nanopore inner sphere enhancement effect on cation adsorption: Sodium and nickel. Soil Science Society of America Journal, 75, 378–388.
Siroux, B., Beaucaire, C., Tabarant, M., Benedetti, M. F., & Reiller, P. E. (2017). Adsorption of strontium and caesium onto an Na-MX80 bentonite: Experiments and building of a coherent thermodynamic modelling. Applied Geochemistry, 87, 167–175.
Shi, J., Liu, H., Lou, Z., Zhang, Y., Meng, Y., Zeng, Q., & Yang, M. (2013). Effect of interlayer counterions on the structures of dry montmorillonites with Si4+/Al3+ substitution. Computational Materials Science, 69, 95–99.
Sposito, G. (2008). The chemistry of soils. Oxford University Press.
Teppen, B. J., & Miller, D. M. (2006). Hydration energy determines isovalent cation exchange selectivity by clay minerals. Soil Science Society of America Journal, 70, 31–40.
Tertre, E., Beaucaire, C., Coreau, N., & Juery, A. (2009). Modelling Zn (II) sorption onto clayey sediments using a multi-site ion-exchange model. Applied Geochemistry, 24, 1852–1861.
The Clay Minerals Society (2019). Physical and chemical data of source clays, http://www.clays.org/sourceclays_data.html, viewed 7 June 2019.
Tournassat, C., Neaman, A., Villiéras, F., Bosbach, D., & Charlet, L. (2003). Nanomorphology of montmorillonite particles: Estimation of the clay edge sorption site density by low-pressure gas adsorption and AFM observations. American Mineralogist, 88, 1989–1995.
Tsipursky, S. I., & Drits, V. A. (1984). The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites studied by oblique-texture electron diffraction. Clay Minerals, 19, 177–193.
Tunega, D., Goodman, B. A., Haberhauer, G., Reichenauer, T. G., Gerzabek, M. H., & Lischka, H. (2007). Ab initio calculations of relative stabilities of different structural arrangements in dioctahedral phyllosilicates. Clays and Clay minerals, 55, 220–232.
Vantelon, D., Montarges-Pelletier, E., Michot, L. J., Pelletier, M., Thomas, F., & Briois, V. (2003). Iron distribution in the octahedral sheet of dioctahedral smectites. An Fe K-edge X-ray absorption spectroscopy study. Physics and Chemistry of Minerals, 30, 44–53.
Viani, A., Gualtieri, A. F., & Artioli, G. (2002). The nature of disorder in montmorillonite by simulation of X-ray powder patterns. American Mineralogist, 87, 966–975.
Wolters, F., Lagaly, G., Kahr, G., Nueeshch, R., & Emmerich, K. (2009). A comprehensive characterization of dioctahedral smectites. Clays and Clay Minerals, 57, 115–133.
Yariv, S. (1992). The effect of tetrahedral substitution of Si by Al on the surface acidity of the oxygen plane of clay minerals. International Reviews in Physical Chemistry, 11, 345–375.
Acknowledgments
This work was supported by the USDA National Institute of Food and Agriculture, Hatch project accession number 1013470.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 157 kb)
Rights and permissions
About this article
Cite this article
Li, Y.W., Schulthess, C.P., Co, K. et al. Influence of Octahedral Cation Distribution in Montmorillonite on Interlayer Hydrogen Counter-Ion Retention Strength via First-Principles Calculations. Clays Clay Miner. 67, 439–448 (2019). https://doi.org/10.1007/s42860-019-00038-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-019-00038-9