Abstract
Thermogravimetric analysis combined with mass spectrometry was used to study H2O bound to samples of illite-1M, illite-2M2 and leucophyllite-1M. Samples were heated in a helium atmosphere at different temperatures and after heating at each given temperature were cooled to 35°C. Each cycle in the mass 18 spectrum of each illite sample contains a low-temperature peak at 60–80°C, a medium-temperature peak at 340–360°C, and a high-temperature peak at a temperature that is very close to the maximum temperature of sample heating of a given cycle. Within each heating-cooling cycle, the sample weight at the beginning of cooling is lower than that at the end of the same cooling stage because of H2O resorption. However, the number of H2O molecules released during each medium-temperature heating cycle is equal to the number of H2O molecules resorbed during the corresponding cooling stages.
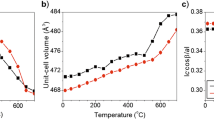
The weight losses, under medium-temperature heating, of the illite samples are related to dehydration when H2O molecules located in K-free sites of the illite interlayers are removed. The medium-temperature peak is reproducible for each cycle because during each cooling stage the illite interlayers resorb the same number of H2O molecules that were lost during the preceding dehydration.
Two distinct features are characteristic of leucophyllite during heating-cooling treatments. First, the number of H2O molecules resorbed during cooling is significantly greater than the number of H2O molecules lost during dehydration. Second, the medium-temperature peaks in the spectrum appear only in the last five cycles and the maximum-peak temperature is 450–460°C. These data indicate that the heating-cooling treatments are accompanied by partial rehydroxylation. This rehydroxylation occurs during each coolingstage when a small number of resorbed H2O molecules are trapped in the interlayers, although most migrate into the octahedral sheet of the 2:1 layers and reform as OH groups. The crystal chemical factors responsible for the dehydration and rehydration as well as for the rehydroxylation reactions are discussed and speculation about the origin of the low- and medium-temperature H2O losses is presented.
Similar content being viewed by others
References
Bailey, S.W. (1984) Crystal chemistry of the true micas. Pp. 13–60 in: Micas (S.W. Bailey, editor). Reviews in Mineralogy, 13. Mineralogical Society of America, Washington, D.C.
Besson, G. and Drits, V.A. (1997) Refined relationships between chemical composition of dioctahedral fine-grained mica minerals and their infrared spectra within the OH stretching region. Part I: Identification of the OH stretching bands. Clays and Clay Minerals, 45, 158–169.
Bish, D.L. and Duffy, C.J. (1990) Thermogravimetric analysis of minerals. Pp. 95–157 in Thermal Analysis in Clay Science (J.W. Stucki and D.L. Bish, Technical Editors, F.A. Mumpton, Managing Editor). CMS Workshop Lectures, Vol. 3, The Clay Minerals Society, Bloomington, Indiana.
Drits, V.A. (2003) Structural and chemical heterogeneity of layer silicates and clay minerals. Clay Minerals, 38, 403–432.
Drits, V.A., Besson, G. and Muller, F. (1995) Structural mechanism of dehydroxylation of cis-vacant 2:1 layer silicates. Clays and Clay Minerals, 43, 718–731.
Drits, V.A., Środoń, J. and Eberl, D.D. (1997) XRD measurement of mean crystallite thickness of illite and illite/smectite: Reappraisal of the Kübler index and the Scherrer equation. Clays and Clay Minerals, 45, 461–475.
Emmerich, K., Madsen, F.T. and Kahr, G. (1999) Dehydroxylation behavior of heat-treated and steam-treated homoionic cis-vacant montmorillonites. Clays and Clay Minerals, 47, 591–604.
Ferrage, E., Lanson, B., Malikova, N., Plançon, A., Sakharov, B.A. and Drits, V.A. (2005) News insights in the distribution of interlayer H2O molecules in bi-hydrated smectite from X-ray diffraction profile modelingof 00l reflections. Chemistry of Materials, 17, 3499–3512.
Grim, R.E. (1968) Clay Mineralogy: International Series in the Earth and Planetary Sciences (F. Press, editor). McGraw-Hill Book Company, New York, 596 pp.
Guggenheim, S. (1990) The dynamics of thermal decomposition in aluminous dioctahedral 2:1 layer silicates: A crystal chemical model. Proceedings of the 9th International Clay Conference, Vol. 2: Surface chemistry, structure and mixed layering of clays (V.C. Farmer and Y. Tardy, editors). Strasbourg, France, pp. 99–107.
Guggenheim, S., Chang, H.Y. and Koster van Groos, A.F. (1987) Muscovite dehydroxylation: High-temperature studies. American Mineralogist, 72, 537–550.
Güven, N. (2001) Mica structure and fibrous growth of illite. Clays and Clay Minerals, 49, 189–196.
Heller, L., Farmer, V.C., Mackenzie, R.C., Mitchell, B.D. and Taylor, H.F.W. (1962) The dehydroxylation and rehydroxylation of triphormic dioctahedral clay minerals. Clay Minerals Bulletin, 5, 56–72.
Heller-Kallai, L. and Rozenson, I. (1980) Dehydroxylation of dioctahedral phyllosilicates. Clays and Clay Minerals, 28, 355–368.
Hower, Y. and Mowatt, G.S. (1966) The mineralogy of illites and mixed-layer illite-montmorillonites. American Mineralogist, 5, 825–854.
Jonas, E.C. (1955) Clays and Clay Minerals (A. Swineford, editor). National Academy of Sciences — National Resource Council, Washington, publication 395, p.66.
Jonas, E.C. and Grim, R.E. (1957) Differential thermal analysis usingcontrolled atmosphere. Pp. 389–403 in: The Differential Thermal Investigation of Clays (R.C. MacKenzie, editor). Monograph 2, Mineralogical Society, London.
Koster van Groos, A.F. and Guggenheim, S. (1987) High-pressure differential thermal analysis (HP-DTA) of the dehydroxylation of Na-rich montmorillonite and K-exchanged montmorillonite. American Mineralogist, 72, 1170–1175.
Koster van Groos, A.F. and Guggenheim, S. (1990) Dehydroxylation of Ca- and Mg-exchanged montmorillonite. American Mineralogist, 74, 627–636.
Mackenzie, R.C. (1957) The Differential Thermal Investigation of Clays. Monograph 2, Mineralogical Society, London, 456 pp.
Mackenzie, R.C. (1982) Down-to-earth thermal analysis. Pp. 25–36 in: Thermal Analysis. (B. Miller, editor). Wiley Heyden Ltd, Chichester, UK.
Mackenzie, R.C., Walker, G.F. and Hart, R. (1949) Illite from Ballater. Mineralogical Magazine, 28, 704–713.
McCarty, D.K. and Reynolds R.C. (2001) Three-dimensional crystal structures of illite-smectite minerals in Paleozoic K-bentonites from the Appalachian Basin. Clays and Clay Minerals, 49, 24–35.
Muller, F., Drits, V.A., Plançon, A. and Besson, G. (2000a) Dehydroxylation of Fe3+, Mg-rich dioctahedral micas: (I) structural transformation. Clay Minerals, 35, 491–504.
Muller, F., Drits, V.A., Tsipursky, S.I. and Plançon, A. (2000b) Dehydroxylation of Fe3+, Mg-rich dioctahedral micas: (II) cation migration. Clay Minerals, 35, 505–514.
Muller, F., Drits, V.A., Plançon, A. and Robert, J.P. (2000c) Structural transformation of 2:1 dioctahedral layer silicates during dehydroxylation-rehydroxylation reactions. Clays and Clay Minerals, 48, 572–585.
Slonimskaya, M.V., Drits, V.A., Finko, V.I. and Salyn, A.L. (1978) The nature of interlayer water in fine-dispersed muscovites. Izvestiya Akademii Nauk SSSR, seriya geologicheskaya, 10, 95–104 (in Russian).
Sokolova, T.N., Drits, V.A., Sokolova, A.L. and Stepanova, K.A. (1976) Structural and mineralogical characteristics and formation conditions of leucophyllite from salt deposits. Litologiya I poleznie iscopaemie, 26, 80–95 (in Russian).
Środoń, J., Elsass, F., McHardy, W.J. and Morgan, D.J. (1992) Chemistry of illite-smectite inferred from TEM measurements of fundamental particles. Clay Minerals, 27, 137–158.
Tsipursky, S.I., Kameneva, M.Y. and Drits, V.A. (1985) Structural transformation of Fe3+-containing 2:1 dioctahedral phyllosilicates in the course of dehydration. 5th Meeting of the European Clay Groups (J. Konta, editor) Prague, pp. 569–577.
Udagawa, S., Urabe, K. and Hasu, H. (1974) The crystal structure of muscovite dehydroxylate. Japanese Association of Mineralogy, Petroleum and Economic Geology, 69, 381–389.
Wardle, R. and Brindley, G.W. (1972) The crystal structures of pyrophyllite-1Tc and of its dehydroxylate. American Mineralogist, 57, 732–750.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drits, V.A., McCarty, D.K. The nature of structure-bonded H2O in illite and leucophyllite from dehydration and dehydroxylation experiments. Clays Clay Miner. 55, 45–58 (2007). https://doi.org/10.1346/CCMN.2007.0550104
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2007.0550104