Abstract
Background
In 2007, the National Quality Forum (NQF) released four performance measures for the treatment of breast cancer. We proposed to study the degree of adherence with these measures among participating institutions in a multi-institutional trial.
Methods
American College of Surgeons Oncology Group (ACOSOG) Z0010 enrolled breast cancer patients onto a phase II trial studying the prognostic significance of bone marrow and sentinel node micrometastases. The current study used χ2 analyses to determine the degree of adherence with four NQF measures among three institution types: academic, community, and teaching affiliate.
Results
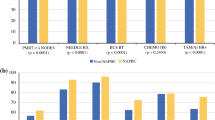
The study revealed small but important differences in two measures. Ninety-five percent of patients from teaching affiliated institutions received whole-breast radiation compared to 92% at academic and 91% at community hospitals. Among patients who were underinsured or uninsured, a marked decrease in radiation use was noted in comparison to patients with insurance—85 versus 93%, respectively. The study also revealed a difference among institutional types in patients undergoing excisional biopsy for diagnosis. In teaching-affiliated hospitals, 28.6% underwent excisional biopsy as compared to 36.8 and 37.4% in academic and community hospitals, respectively. There was no statistically significant difference between adherence rates with the remaining two measures. Adjuvant chemotherapy was administered to patients with hormone receptor negative tumors ≥1 cm in size in 79–85% of institutions. Tamoxifen was administered to 79–82% of those patients with hormone receptor–positive cancers.
Conclusions
Among breast cancer patients enrolled onto a multi-institutional clinical trial, we found a high degree of adherence with current consensus standards for adjuvant treatment, despite varied practice environments.
Similar content being viewed by others
References
American Cancer Society. http://www.cancer.org/downloads/STT/Global_Facts_and_Figures_2007rev2.pdf. Accessed October 12, 2009.
American Society of Clinical Oncologists. http://www.qualityforum.org/. Accessed October 12, 2009.
American Society of Breast Surgeons. http://breastsurgeons.org/. Accessed October 12, 2009.
National Accreditation Program for Breast Centers. http://accreditedbreastcenters.org/. Accessed October 12, 2009.
Hassett MJ, Hughes ME, Niland JC, et al. Selecting high priority quality measures for breast cancer quality improvement. Med Care. 2008;46:762–70.
National Quality Forum. http://www.qualityforum.org/. Accessed October 12, 2009.
Cheng SH, Wang CJ, Lin JL, Horng CF, Lu MC, Asch SM, et al. Adherence to quality indicators and survival in patients with breast cancer. Med Care. 2009;47:217–25.
Schacter AM, Mamaladze V, Lewin G, Graham ID, Brouwers M, Sampson M, et al. Many quality measurements, but few quality measures assessing the quality of breast cancer care in women: a systematic review. BMC Cancer. 2006;6:291.
Brucker SY, Schumacher C, Sohn C, Rezai M, Bamberg M, Wallwiener D. (2008) Steering Committee. Benchmarking the quality of breast cancer care in a nationwide voluntary system: the first five-year results (2003–2007) from Germany as a proof of concept. BMC Cancer. 8:358.
Mandelblatt JS, Potosky AL. On the road to improving the quality of breast cancer care: a distance still to travel. Med Care. 2008;46:759–61.
Malin JL, Schneider EC, Epstein AM, et al. Results of the National Initiative for Cancer Care Quality. How can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24:626–33.
Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network quality measures. J Clin Oncol. 2008;26:3631–7.
National Quality Forum. NQF-endorsed standards. http://www.qualityforum.org/Measures_List.aspx?keyword=breast+cancer. Accessed October 12, 2009.
Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500.
Leitch AM, Beitsch PD, McCall LM, et al. Patterns of participation and successful patient recruitment to American College of Surgeons Oncology Group Z0010, a phase II trial for patients with early-stage breast cancer. Am J Surg. 2005;190:539–42.
Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005;242:593–9.
American College of Surgeons. http://www.facs.org/cancer/publicncdb.html. Accessed October 12, 2009.
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41.
Veronesi U, Cascinelli N, Mariani L. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717.
Smith BD, Smith GL, Roberts KB, et al. Baseline utilization of breast radiotherapy before institution of the Medicare practice quality reporting initiative. Int J Radiat Oncol Biol Phys. 2009;74:1506–12.
Buchholz TA, Theriault RL, Niland JC, et al. The use of radiation as a component of breast conservation therapy in National Comprehensive Cancer Network centers. J Clin Oncol. 2006;24:361–9.
Acknowledgment
This work was supported by funding from the NCI ACOSOG U10 CA 76001. We thank the all of the investigators and their site research teams, and in particular the patients with breast cancer and their caregivers who participated in the Z0010 study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilke, L.G., Ballman, K.V., McCall, L.M. et al. Adherence to the National Quality Forum (NQF) Breast Cancer Measures Within Cancer Clinical Trials: A Review From ACOSOG Z0010. Ann Surg Oncol 17, 1989–1994 (2010). https://doi.org/10.1245/s10434-010-0980-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-0980-9