Abstract
Introduction
The Commission on Cancer (CoC) issues Cancer Program Practice Profile Reports (CP3R) that set standards for high-quality care. Three metrics for breast cancer include radiation within 1 year for women < 70 years of age receiving breast-conserving surgery, radiation within 1 year after mastectomy for women with four or more positive lymph nodes (MASTRT), and hormonal therapy within 1 year of a stage IB–III hormone receptor-positive breast cancer (HT). Our study evaluates national trends in quality metric compliance.
Methods
The National Cancer Database was queried from 2004 to 2014 to identify patients who met the criteria for the three quality metrics. National trends in compliance were compared.
Results
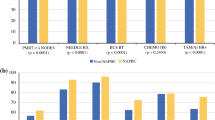
Overall, 1,094,264 patients qualified for BCSRT (n = 534,147), MASTRT (n = 66,291), or HT (n = 493,826). In 2014, 91.1% of patients met BCSRT, 88.4% met MASTRT, and 90.7% met HT. BCSRT, MASTRT, and HT compliance rates were lower in community hospitals compared with Integrated Network Cancer Programs (INCP) (BCSRT: 89.0% vs. 92.8%, p < 0.01; MASTRT: 85.5% vs. 90.6%, p < 0.01; HT: 87.3% vs. 93.7%, p < 0.01). On multivariate analysis, patients receiving care at an INCP facility [odds ratio (OR) 1.47, 95% confidence interval (CI) 1.37–1.58] and insured patients (OR 1.70, 95% CI 1.54–1.87) had higher odds of BCSRT compliance, and minorities (OR 0.76, 95% CI 0.73–0.80) had lower odds. Similar results were seen for MASTRT and HT.
Conclusion
In more recent years, overall compliance rates for breast cancer quality metrics of BCSRT and HT by Comprehensive Community Cancer Programs, Academic/Research Programs, and INCPs have increased to meet the 90% CoC standards, while MASTRT has regressed. Community programs were least compliant with meeting the CoC standards.

Similar content being viewed by others
References
American College of Surgeons. National Cancer Database. Available at: https://www.facs.org/quality-programs/cancer/ncdb/qualitymeasures. Accessed 30 Apr 2020.
Miller ME, Bleicher RJ, Kaufman CS, et al. Impact of breast center accredidation on compliance with breast quality performance measures at commission on cancer-accredited centers. Ann Surg Oncol. 2019;26:1202–11.
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001.
Parekh A, Fu W, Hu C, et al. Impact of race, ethnicity, and socioeconomic factors on receipt of radiation after breast conservation surgery: analysis of the national cancer database. Breast Cancer Res Treat. 2018;172(1):201–8.
Chu QD, Caldito G, Miller JK, Townsend B, et al. Postmastectomy radiation for N2/N3 breast cancer: factors associated with low compliance rate. J Am Coll Surg. 2014;220(4):659–69.
McGinnis LS, Menck HR, Eyre HJ, et al. National cancer data base survey of breast cancer management for patients from low income zip codes. Cancer. 2000;88(4):933–45.
Cancer Programs Practice Profile Reports (CP3R) Rapid Quality Reporting System (RQRS). https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/measure%20specs%20breast.ashx. Accessed 2 Jul 2020.
Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of cancer. N Engl J Med. 1985;312:665–73.
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41.
Reyes SA, Williams AD, Arlow RL, et al. Changing practice patterns of adjuvant radiation among elderly women with early stage breast cancer in the United States from 2004 to 2014. Breast J. 2020;26(3):353–67.
Haque W, Verma V, Hsiao K, et al. Omission of radiation therapy following breast conservation in older (≥70 years) women with T1–2N0 triple-negative breast cancer. Breast J. 2019;25(6):1126–33.
EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomized trials [published erratum appears in Lancet. 2014 Nov;384(9957):1848]. Lancet. 2014;383(9935):2127-2135.
Ohri N, Sittig MP, Tsai CJ, et al. Trends and variations in postmastectomy radiation therapy for breast cancer in patients with 1 to 3 positive lymph nodes: A national cancer data base analysis. Cancer. 2018;124(3):482–90.
Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet. 2013;381:805–16.
Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69.
Regan MM, Francis PA, Pagani O, et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trials. J Clin Oncol. 2016;34(19):2221–31.
Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group; Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet. 2008;9:45–53.
McGinnis LS, Menck HR, Eyre HJ, Bland KI, Scott-Conner CE, Morrow M, Winchester DP. National cancer data base survey of breast cancer management for patients from low income zip codes. Cancer. 2000;88(4):933–45.
Lodrigues W, Dumas J, Rao M, Lilley L, Rao R, et al. Compliance with the Commission on Cancer Quality of Breast Cancer Care Measures: Self-Evaluation Advised. Breast J. 2011;17(2):167–71.
Acknowledgments
The authors thank the Fashion Footwear Charitable Foundation of New York, Inc.; The Margie and Robert E. Petersen Foundation; Associates for Breast and Prostate Cancer Studies; and Linda and Jim Lippman.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Alice Chung has received a grant from Agendia, Inc. for research unrelated to the work in the submitted manuscript. Marissa K. Srour, Joshua Tseng, Armando E. Giuliano, and Farin Amersi have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srour, M.K., Tseng, J., Chung, A. et al. Contemporary Cancer Program Practice Profile Report (CP3R) Compliance Rates for Breast Cancer: A National Cancer Database Analysis. Ann Surg Oncol 28, 8589–8599 (2021). https://doi.org/10.1245/s10434-021-10028-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10028-8