Abstract
Cannabinoids, such as ∆9-tetrahydrocannabinol (THC) and cannabidiol (CBD), are effective bioactive compounds that improve the quality of life of patients with certain chronic conditions. The copolymer poly(lactic-co-glycolic acid) (PLGA) has been used to encapsulate such compounds separately, providing pharmaceutical grade edible products with unique features. In this work, a variety of PLGA based nanoformulations that maintain the natural cannabinoid profile found in the plant (known as full-spectrum) are proposed and evaluated. Three different cannabis sources were used, representing the three most relevant cannabis chemotypes. PLGA nanocapsules loaded with different amounts of cannabinoids were prepared by nanoemulsion, and were then functionalized with three of the most common coating polymers: pectin, alginate and chitosan. In order to evaluate the suitability of the proposed formulations, all the synthesized nanocapsules were characterized, and their cannabinoid content, size, zeta-potential, morphology and in vitro bioaccessibility was determined. Regardless of the employed cannabis source, its load and the functionalization, high cannabinoid content PLGA nanocapsules with suitable particle size and zeta-potential were obtained. Study of nanocapsules’ morphology and in vitro release assays in gastro-intestinal media suggested that high cannabis source load may compromise the structure of nanocapsules and their release properties, and hence, the use of lower content of cannabis source is recommended.
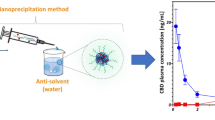
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The therapeutic strength of Cannabis Sativa L. has been well-known in various cultures from 3000 BC to the present days [1]. The medicinal properties of the plant comes from the various bioactive compounds that have their own palm, such as, terpenes, flavonoids, alkaloids and, most importantly, cannabionids, which are specific of the Cannabis plant [1, 2]. Cannabinoids interfere in the endocannabinoid system, favoring a variety of psychological and physiological effects due to the different affinities between cannabinoids and endocannabinoid system’s receptors (mainly CB1 and CB2) [2, 3].
Among the various cannabinoids (more than 120) identified in the literature, ∆9-tetrahydrocannabinol (∆9-THC or THC) and cannabidiol (CBD) still are the most known and widely used ones, since they are the major cannabinoids in most cannabis plants and they have shown to be effective to relief the symptoms of chronic pain and other diseases [4]. THC is widely known for its psychoactivity, but also for its analgesic, anti-inflammatory, appetite stimulant and antiemetic properties [5]. CBD, has shown also analgesic and anti-inflammatory effects, together to anxiolytic, anti-epilepsy and antipsychotic effects [6]. Because they have such diverse effects, the choice between THC or CBD is made depending on the therapeutic application, although the combination of both has proven to be practical in reducing the side effects of THC (i.e., toxic psychosis, dizziness, dry mouth…), so the combination of both is often sought [7]. Besides THC and CBD, minor cannabinoids such as cannabigerol (CBG), cannabichromene (CBC), cannabinol (CBN) and tetrahydrocannabivarin (THCV), have demonstrated therapeutic efficacy for different applications, although the main interest towards these cannabinoids is their use as enhancers of the effects of major cannabinoids [8]. As with the combination of THC and CBD, the simulatenous use of multiple cannabinoids can improve the therapeutic benefits of major cannabinoid. Indeed, it is believed that consuming the full spectrum of cannabinoids present in the plant may promote a synergistic effect or “entourage” effect of major cannabinoids. For this reason cannabis-derived products that maintain the cannabinoid composition of the plant (usually referred to as full-spectrum products) have gained interest in the recent years [5, 8, 9]. Related to this, the extended knowledge in the plant, including genetics and cultivation conditions, has led to the option to develop a vast variety of Cannabis varieties, each of them having a unique spectrum of cannabinoids [10].
Nowadays, a wide variety of cannabis-derived products are available ranging from vaporizable plant extracts to creams and lotions. Nevertheless, when it comes to medicinal use, products designed for oral administration are preferred because of their ease of dosing and self-medication, their reduced toxicity and the ability of providing long-lasting effects [7, 11, 12]. The mayor drawback faced by edible products is the low oral bioavailability of cannabinoids. This is partly due to the lipophilic nature of cannabinoids (log P ~ 6–7), which makes them to have a poor solubility in aqueous media (2–10 μg/mL) and negatively affects their absorption in the intestine [13, 14]. Additionally, cannabinoids have low stability in acidic and oxidative environments, especially in the presence of light and heat [15, 16]. Therefore, cannabinoids can easily be degraded through the digestive tract, particularly in the stomach [17]. The matrix in which cannabinoids are found can play a crucial role in improving their solubility and physicochemical stability, and hence, their pharmacokinetics and bioavailability [18]. Encapsulation of cannabinoids in polymeric vehicles has proved to be a suitable strategy for trapping cannabinoids in matrixes that provide enhanced oral bioavailability, while offering the possibility to design formulations with specific release profiles [18,19,20]. In this regard, polymeric nanoformulations have emerged as a promising tool to provide a targeted delivery of cannabinoids, promote improved oral bioavailability and desired pharmacokinetic profiles [20,21,22,23].
Within the wide range of polymeric materials that can be used to elaborate nanocarriers, poly(lactic-co-glycolic acid) (PLGA) can be highlighted due to its: (i) biodegradability and biocompatibility, (ii) ability to provide a sustained, controlled and targeted release, and, (iii) versatility in terms of routes of administration (e.g., oral, topical, ocular, transdermal) among other properties [24,25,26,27]. Those properties have led to PLGA as one of the most used polymeric material to design pharmaceuticals containing a wide variety of bioactive compounds [25, 27]. For instance, PLGA-based nanoformulations have recently been proposed to encapsulate various drugs and bioactive compounds, such as coumarin C75, oxaliplatin, Leishmania infantum antigens and quercetin. The use of PLGA nanocarriers has improved solubility and cellular uptake of such compounds, and in general, increased the efficacy of their pharamacological action [28,29,30,31].
In the field of cannabinoids, Fraguas-Sánchez and coworkers, developed CBD-loaded PLGA injectable nanoparticles for the treatment of different types of cancer (ovarian and breast) with very promising results [32, 33]. Likewise, the research group of Martin-Banderas has developed a series of PLGA nanoformulations loaded with CB13 (a synthetic cannabinoid derivative) designed for oral administration. These formulations have shown to offer an improved intestinal absorption and biodistribution of cannabinoids, sustained long-term (10–14 days) release of cannabinoids, and minimal cytotoxicity, making them promising formulations for treating chronic pain, among other potential uses [34,35,36,37].
Nonetheless, it has been demonstrated that the addition of an outer layer (or coating) by the functionalization of PLGA nanocapsules, using different polymers can even further improve their characteristics in terms of absorption, biodistribution and therapeutic efficacy [30, 34,35,36,37,38,39]. For instance, chitosan is a natural cationic polysaccharide that has been widely used to functionalize PLGA nanoparticles. The inclusion of a chitosan coating can significantly improve intestinal permeability and absorption of PLGA nanoparticles, as it is able to open tight junctions in the intestinal epithelium [40,41,42,43,44]. Despite the proven efficacy of chitosan, other well-known natural polysaccharides, such as alginate and pectin, can also be used to improve the features of PLGA nanoparticles [45]. Pectin and alginate are anionic polymers, unlike chitosan, are more stable at low pH values and are therefore used for intestinal and colonal drug delivery [44]. Pectin is commonly used to enhance shelf-life of encapsualted bioactive compounds, prevents irritation of the intestinal mucosa, and can improve intestinal retention, intestinal mucoadhesivity and transport properties of capsules [44, 46,47,48]. Similarly, alginate is a common coating that can improve encapsulation efficiency, integrity of capsules through the gastrointestinal track, and their mucoadhesivity, so it has been used previously to improve release and absorption properties of PLGA nanoformulations [38, 49,50,51].
In this context, and highlighting the fact that the cannabinoid-PLGA formulations developed to date only contain THC, CBD or CB13 [23, 52], a variety of PLGA based nanoformulations that maintain the natural cannabinoid profile found in the plant, or full-spectrum formulations, are proposed and evaluated in the present work. For this main goal, three cannabis strains, containing different cannabinoid profiles, were used, and non-functionalized PLGA capsules together with pectin, alginate or chitosan coated capsules were prepared with each of them. In order to evaluate the overall features of the proposed formulations, to rule out possible inadequate formulations and to have a preliminary idea of their possible behavior in case of ingestion, a basic physicochemical characterization was carried. Thus, the cannabinoid content, particle-size, morphology and zeta-potential of the proposed formulatios were evaluated, and the potential release profile and oral bioaccessibility of entrapped cannabinoids were studied by simulated in vitro digestion.
Materials and Methods
Materials and Standards
The PLGA (powder, 50:50) employed for the synthesis of nanocapsules was obtained from MedChemExpress (Monmouth Junction, NJ, USA). Polyvinyl alcohol (PVA, 13.000—23.000 g/mol, 98% hydrolyzed), alginic acid sodium salt from brown algae (low viscosity), chitosan (medium molecular weight), sucrose (for molecular biology, ≥ 99.5%), pepsin from porcine gastric mucosa (powder, ≥ 250 units/mg solid, lot result: 444 U/mg), pancreatin from porcine pancreas (powder, suitable for cell culture, 4 × USP specifications), bile salts (for microbiology) and sodium bicarbonate (ACS reagent, ≥ 99.7%) were purchased from Sigma-Aldrich Chemie GmbH (Schnelldorf, Germany). Pectin was produced by Guinama (La Pobla de Vallbona, Spain) and phosphatidylcholine (Phospholipon® 90 G, ≥ 94%) by Phospholipid GmbH (Cologne, Germany). Hydrochloric acid (HCl, 36%) was purchased from Merck KGaA (Dramstadt, Germany). Ethanol (99.5%) was obtained from Panreac Química S.L.U. (Barcelona, Spain) and ethyl acetate (≥ 99.8%, ChromAR® for HPLC) and acetonitrile (≥ 99.8%, ChromAR® for HPLC) from Macron Fine Chemicals (Gliwice, Poland). Activated charcoal (powder, reagent grade), methanol (UHPLC-MS grade) and water (UHPLC-MS grade) were purchased from Sharlab (Sentmenat, Spain), whereas Milli-Q quality water (< 0.05 µS∙cm−1) was produced using a Millipore 185 from Millipore (Burlington, MA, USA).
The solutions of individual standards of cannabinoids ∆9-tetrahydrocannabinolic acid-A (THCA, 1000 µg/mL in methanol), ∆9-tetrahydrocannabinol (THC, 1000 µg/mL in methanol) ∆9-tetrahydrocannabivarin (THCV, 1000 µg/mL in acetonitrile), ∆9-tetrahydrocannabivarinic acid (THCVA, 1000 µg/mL in acetonitrile), cannabichromene (CBC, 1000 µg/mL in methanol), cannabichromenic acid (CBCA, 1000 µg/mL in acetonitrile), cannabidiol (CBD, 1000 µg/mL in methanol), cannabidiolic acid (CBDA, 1000 µg/mL in acetonitrile), cannabigerol (CBG, 1000 µg/mL in methanol), cannabigerolic acid (CBGA, 1000 µg/mL in acetonitrile), cannabinol (CBN, 1000 µg/mL in methanol) and cannabinolic acid (CBNA, 1000 µg/mL in methanol), cannabidivarin (CBDV, 1000 µg/mL in methanol), cannabidivarinic acid (CBDVA, 1000 µg/mL in acetonitrile) were purchased by Dr. Ehrenstofer GmbH (Augsburg, Germany). The deuterated analogue ∆9-tetrahydrocannabinol (THC, 1000 µg/mL in methanol) was supplied by Merck KGaA (Dramstadt, Germany) and phenantrene, used as internal standard (IS), was purchased from Sigma-Aldrich Chimie (Saint-Quentin-Fallavier). A mixed fresh stock solution containing 100 μg/mL of all target compounds was prepared monthly in methanol, whereas intermediate dilutions were prepared daily according to the experimentation.
Preparation of Purified Cannabis Extracts
Cannabis flowers from three different cannabis strains were made into enriched cannabis extracts. The strains were chosen to be representative of the three most relevant cannabis chemotypes: (i) Chemotype I (CI) – a THC rich strain (~ 12% THC), (ii) Chemotype II (CII) – a THC/CBD leveled strain (~ 5% THC and ~ 7% CBD) and (iii) Chemotype III (CIII) – a CBD rich strain (~ 11% CBD). Flowers (around 15 g) of each strain were grinded and placed into separate closed Pyrex containers, and were maintained at 120 °C for 1 h to assure complete decarboxylation of acidic cannabinoids while keeping loses to a minimum [53]. After that, the Pyrex containers were stored at –40 °C, and once they were cooled down, cold ethanol (–40 °C) was added until the decarboxylated flowers were fully covered. Cold extraction of neutral cannabinoids was performed by maintaining the ethanol solutions in an ultrasonic bath for 1 min and, then 5 min at –40 °C. Once extraction was completed, ethanol extracts were vacuum filtered using 10—12 µm cellulose filters, and the resulting extracts were stored at –80 °C overnight to purify them by winterization [54]. The waxes, and lipids that precipitated during this period, were removed by a fast vacuum filtration using 0.45 µm cellulose nitrate filters. The remaining chlorophyll was removed by adding 80 mg of activated charcoal per initial cannabis flower gram (1.25 g for each extract approximately), vortexing for 10 min and filtering using 0.45 µm polypropylene syringe disk filters. Finally, the excess of ethanol was removed by a rotary evaporator (35 °C, 100 mbar) until viscous extracts were obtained (around 2 g per strain). The three extracts were stored at –20 °C until further use.
Cannabinoid Content in Purified Cannabis Extracts
The concentration of main cannabinoids of each cannabis strain’s extract was determined by means of high performance liquid chromatography coupled to a diode array detector (HPLC–DAD) in triplicate following a previously validated method [55]. Briefly, 0.1 g of cannabis extract were sonicated with 5 mL of methanol in an ultrasonic bath for 15 min. Subsequently, the solution was centrifuged for 5 min at 10000 g in a 5804 R Eppendorf centrifuge (Hamburg, Germany), and the supernatant solution was diluted with methanol containing IS (resulting in a concentration of 5 µg/mL IS in the final solution). The final solutions were syringe-filtered with 0.22 µm polypropylene disks and analyzed by means of HPLC–DAD using the method described in the following sections.
Preparation of PLGA Nanoparticles
PLGA nanoparticles were prepared based on an emulsion solvent evaporation technique previously described for encapsulation of pure CBD [33] with some modifications due to the nature of the cannabis extracts. First, 12 PLGA solutions, each of them containing 500 mg of PLGA dissolved in 10 mL of ethyl acetate, were prepared. The PLGA-ethyl acetate solutions were loaded with cannabinoids by adding the different cannabis extracts. Owing to the lack of previous references related to cannabis extract containing PLGA formulations, four cannabis extract to PLGA powder mass ratios (referred as load ratio from now on) were tested for each of the cannabis strains (see Table I). Depending on this, 50, 150, 300 or 500 mg of each strain’s extract were added to each of the 12 initial PLGA solutions (resulting in 4 loading ratios per strain). Once the extracts were dissolved, the PLGA-ethyl acetate-cannabis solutions were added dropwise into 50 mL of PVA 0.5% aqueous solutions, which were stirred at 24000 rpm by an Ultra-turrax (IKA, Staufen, Germany) in order to emulsify nanoparticles. This process was carried out for all formulations except for the highest loading ratio (i.e., 10:10) since an indivisible agglomerate that blocked the Ultra-turrax was formed during the process. After emulsification, excess of ethyl acetate was removed by rotary evaporation (40 °C, 100 mbar) while excess of PVA and non-ecapsulated cannabinoids were removed by centrifugation and decantation for three times by adding further Milli-Q water. The obtained wet PLGA-cannabis nanoparticles for each strain and load were separated in four aliquots for further functionalization. One of the aliquots per load and strain was stored at 4 °C (uncoated capsules) and the other three were functionalized using different coating layers: alginate, pectin and chitosan (see Table I). The functionalization of those PLGA-cannabis nanoparticles was carried out by the addition of 10 mL of 0.2% aqueous solutions of the corresponding coating agent (i.e., sodium alginate, pectin, and chitosan) onto each of the aliquots and continuously agitated at room temperature for 2 h. Then, Milli-Q water was added and the excess of coating polymer, along with potential traces of non-encapsulated cannabinoids were removed through centrifugation and decantation. This process was repeated three times by adding further Milli-Q water. Once cleaned, 10 mL of cryoprotecting solution (i.e., sucrose 1% aqueous solution [56]), was added to each of the 4 fractions (non-functionalized and functionalized) for each strain and load; and were dried subsequently in a Coolvacuum Lyomicron freeze-dryer (Barcelona, Spain) at –60 °C and 0.037 mbar for 48 h. The sucrose was separated and the obtained dry capsules were weighed and stored at –20 °C until further use.
Cannabinoid Content in PLGA Nanoparticles
The concentration of 14 cannabinoids in the synthetized PLGA-cannabinoid nanoparticles was determined in triplicate by means of HPLC–DAD following a method described in the literature used to determine the content of a synthetic cannabinoid in PLGA nanoparticles [37]. 1 mL of acetonitrile was added onto accurately weighed 4 mg of lyophilized PLGA nanoparticles, and the mixture was vortexed (5 min) to dissolve PLGA nanoparticles and promote liberation of entrapped compounds in the organic phase [37, 57,58,59]. The obtained acetonitrile solutions were diluted in methanol containing IS (resulting in a concentration of 5 µg/mL in the final solutions) and filtered using 0.22 µm polypropylene disks. The cannabinoid content in the final solutions was determined by means of HPLC–DAD analyisis.
5 µL of the final solutions were analyized using an Infinity 1260 LC System (HPLC) coupled to an Infinity 1260 Diode Array Detector WR (DAD), both from Agilent Technologies (Santa Clara, CA, USA). Separation of cannabinoids was achieved using a Kinetex C18 column (150 × 3 mm, 2.6 µm) with a Security Guard Ultra C18 precolumn (2 × 3 mm), both from Phenomenex (Torrance, CA, USA). The separation of cannabinoids was done using a gradient method at a constant flow of 0.7 mL/min consisted of mobile phases A (Water, 0.1% acetic acid) and B (Methanol, 0.1% acetic acid). The gradient method started at 30% A and maintained 3 min; then, it was decreased first to 20% in 6 min and then to 5% in 3 min, which was maintained for 3 min. A was increased to 30% in 5 min and maintained for other 4 min to reach initial conditions before the next chromatographic run, which last 24 min in total. Cannabinoids were detected using DAD and were quantified at 230 nm using an external calibration curve prepared in the range of 0.1 µg/mL and 50 µg/mL for all target compounds. All the calibration solutions, as well as the measured extracts, contained IS in a concentration of 5 µg/mL in order to correct the DAD signal and minimize instrumental measurements variability.
Encapsulation-efficiency
The encapsulation-efficiencies (EE %) for each formulation and cannabinoid were determined by comparing encapsulated mass (mgcap) of each cannabinoid with the mass of cannabinoid that was loaded along with the cannabis extract (mgext) (Eq. 1):
where, mcap is the obtained mass in g of nanoparticles, Ccap is the concentration in mg/g of each cannabinoid in each formulation, mext is the employed mass in mg of cannabis extract and Cext is the concentration of each cannabinoid in mg/g in each of the cannabis extracts.
Physical Characterization of PLGA Nanoparticles: Particle Size, Zeta-Potential and SEM Imaging
The particle size and zeta-potential of PLGA nanocapsules were measured using a Zetasizer Nano series from Malvern Instruments (Malvern, UK). For this purpose, 4 mg of dried PLGA nanocapsules were suspended in 4 mL of Milli-Q water under continuous magnetic stirring to promote homogeneity of the suspension. Aliquots of the homogenous dispersions were used to prepare dilutions of 0.05 mg/mL (particle size) and 0.5 mg/mL (zeta-potential). The particle size and zeta-potential of three aliquots of the final dilutions were determined respectively by dynamic light scattering and electrophoretic light scattering (at an angle of 90°).
The morphology of the synthetized nanocapsules was checked by means of Scanning Electron Microscopy (SEM). The dried PLGA nanocapsules were coated with 15 nm of gold on a K550X sputter coater from Emitech (Montigny-le-Bretonneux, France) and measured using a FEG SEM S4800 from Hitachi (Tokyo, Japan) with an acceleration voltage of 5 kV.
In Vitro Gastro-Intestinal Digestion Simulation
Release of the main cannabinoids was determined by a static in vitro digestion simulation, following the recommendations of INFOGEST [60] and Minekus et al. [61]. Simulated gastric fluid (SGF) contained 2000 U/mL of pepsin and 0.17 mM of phosphatidylcholine in Milli-Q water and the pH was adjusted to 3 using a 2 M HCl solution. Simulated intestinal fluid (SIF) contained 3.2 g/L of pancreatine and 8.16 g/L of bile salts in Milli-Q water. The content of main cannabinoids (THC and CBD) in dry PLGA nanocapsules was onsidered to standardize the assays and provide an easier comparison between formulations. The mass employed in each assay was the one required to provide 10 mg of THC (CI nanoparticles), 10 mg of CBD (CIII nanocapsules) or 10 mg of THC + CBD (CII capsules).
For each formulation the calculated PLGA nanoparticles mass was weighed and 10 mL of SGF were added and maintained at 37 ˚C under continuous stirring for 2 h, simulating the gastric phase. After that, 10 mL of SIF were added (resulting in 1.6 g/L of pancreatine and 4.08 g/L of bile salts), pH was adjusted to 7 using a saturated sodium bicarbonate aqueous solution, and the mixture was maintained at 37 ˚C under continuous stirring for 4 h, simulating the intestinal phase. The release profile of cannabinoids was determined by taking aliquots of 200 µL at different timings (12 aliquots during the 6 h of the experiment). Each time the aliquots were taken, aliquots of 200 µL of fresh SGF or SGF:SIF 1:1 mixture were added to the assay-solution in order to maintain the same ratio within microcapsules and simulated fluids in the whole simulation. All the assays were run in triplicate. All the aliquots were diluted with methanol containing IS (resulting in a concentration of 5 µg/mL IS in the final solution) and centrifuged using a 5424 R Eppendorf Centrifuge (Hamburg, Germany) for 5 min at 10000 g. The supernatants were collected and filtered with 0.22 µm polypropylene syringe disks prior to HPLC–DAD analysis.
The results were expressed as the released cumulative fraction from the total cannabinoid content through digestion time. Intestinal bioavailability was calculated by resting the fraction released in the gastric phase to the final released fraction.
Results
Cannabinoid Content and Encapsulation-efficiency
The content of 14 cannabinoids in the synthetized PLGA nanoformulations is compiled in Table S1. Since the applied polymeric coating did not result in significant differences (p-value > 0.05) in the cannabinoid content of capsules made with the same cannabis strain and extract loading, the mean content of the uncoated and three coated formulations per strain and extract loading are shown in Fig. 1. The cannabinoid content in the obtained PLGA nanocapsules are in line with the cannabinoid profile of the employed extract and the loaded extract amount in each case (cannabinoid content of the extracts are shown in Table S2).
CI capsules, contained high amounts of THC, between 8 and 35% of the capsules’ mass, and contained CBD, CBC, CBG, CBN and THCV (together with their acidic analogs) in concentrations between 0.01% and 0.7%. This made CI capsules to have the highest content of cannabinoids and to have the widest spectrum of neutral cannabinoids, compared to the capsules obtained from CII and CIII strains. CII capsules, contained between 3.8% and 14% of THC and between 4.0% and 15% of CBD, and the content of CBC, CBG and CBN (together with their acidic analogs) were encountered in concentrations between 0.01% and 1.16%. CIII capsules, on the other hand, contained high amounts of CBD, between 6.9% and 26% of the capsules’ mass and contained CBC and CBG in concentrations between 0.01% and 1.3%. Thus, the total cannabinoid content in CII and CIII is slightly lower, and although the cannabinoid spectrum is tighter, the content in minor cannabinoids such as CBC and CBG, is higher than in CI capsules. Considering that the average oral dose is about 30 mg for THC, 60 mg for THC + CBD (30 mg of each) and about 100 mg for CBD [7, 62, 63], between 360 and 80 mg of CI capsules, 790 and 200 mg of CII capsules and 1400 and 400 mg of CIII capsules would be enough to ensure those doses.
In addition to the expected differences related to the nature of the cannabis strain and the extratct load, some similarities can be highlighted. The content of acidic cannabinoids in all the formulations is minimum, especially the content of THCA (even in high THC content capsules) due to its lower decarboxylation temperature [53]. This observation indicates that the decarboxylation process carried in the preparation of the three cannabis extracts was appropiate. Similarly, low content of CBN (the main degradation product of THC’s oxidation) was observed in all capsules, which is a fact of great interest in the case of CI and CII capsules (both containing high amounts of THC). Based on this result, we can conclude that the employed cannabis flowers were fresh (the higher CBN content is, the older a cannabis product is) and that THC was not oxidized neither in the preparation of the cannabis extracts nor in the encapsulation process [64, 65]. This could be achieved by avoiding high temperatures (after decarboxylation), prolonged exposure to light and exposure to oxidant species.
THCVA, CBDV and CBDVA were under the method limits of detection for the measured dilutions (0.01%). Although the concentration of these minor cannabinoids could have been determined by measuring a higher concentration extract, this option was discarded since the required dilution to measure these cannabinoids, based on the low content in the purified extracts (see Table S2), would saturate the column and the HPLC system.
Concerning the encapsulation efficiencies (EE) of major cannabinoids, a slight decrease was observed as the cannabis extract loading was increased in all formulations (Table II). The highest EEs were obtained in nanocapsules loaded with 10 mg of cannabis extract per 100 mg of PLGA, where mean EEs between 85 and 99% were obtained. When the cannabis extract load was increased to 3:10, the mean EE decreased to values between 65 and 84%, and when increased to 6:10, the EE % decreased to values between 56 and 73%. Likewise, Martin-Banderas et al. and Fraguas-Sanchez et.al reported lower EE % of CB13 or CBD in their PLGA based encapsulations when cannabinoid load was increased [33, 37], which is attached to capsules’ maximum loading capacity or to loss of available drug due to its migration into the emulsifier aqueous phase. In contrast to this phenomenon, no significant differences were observed in the EE % of different strains and cannabinoids.
Physical Properties of PLGA Nanocapsules: Particle Size, Zeta-potential and SEM Imaging
Particle size and zeta-potential of PLGA nanoparticles were determined using a zeta-sizer, measurement plots of both parameters are shown in Figures S1 and S2 respectively. The polidispersity index (PDI) values of most formulations were around 0.3 (Table S3), indicating that they had an acceptable dispersity [66]. For this reason, and according to ISO 22412:2017, Z-average was elected to report the mean particle size of each formulation [67]. The particle size ranged between 150 and 700 nm, being the applied coating in each formation the possible responsible of such variability (Fig. 2A). In fact, particles with the same coating showed statistically comparable particle sizes, being the pectin coated capsules the largest ones (380—700 nm) compared to either uncoated (160—440 nm), alginate coated (200—480 nm) or chitosan coated (150—450 nm) capsules (depending on the case). On the contrary, no clear differences were observed among strains and extract load ratios. Uncoated, alginate coated and chitosan coated nanocapsules showed comparable particle sizes among them in most cases. The obtained particle sizes fall into the same size range than those obtained in THC, CBD or CB13 loaded PLGA nanoparticles in previous works, which ranged between 250 and 450 nm in non-functionalized particles and between 600 and 900 nm in functionalized particles [52].
The success in the surface-modification (or coating) process and its effect on the surface-charge of nanoparticles was assessed using the zeta-potential values (Fig. 1b). As expected, uncoated, pectin coated and alginate coated capsules showed negative zeta-potentials due to their anionic nature, whereas chitosan coated capsules showed positive zeta-potentials due to their cationic nature. The addition of pectin and alginate, increased significantly the negative charge of uncoated PLGA capsules in CI 1:10 and 3:10 loaded capsules, CII 3:10 loaded capsules and all CIII capsules. Interestingly, in all cases (except for CIII 1:10 loaded capsules) the zeta-potential of pectin and alginate were comparable between them (p > 0.05), as well as comparable among nanocapsules derived from different extract load ratios and cannabis strains. In the case of uncoated capsules, despite no clear tendency was observed, significant differences were observed among formulations, since CI 1:10 loaded, and CIII capsules showed less negative zeta-potentials compared to the rest. For this reason, surface modification using pectin and alginate, seems a good strategy to increase negative zeta-potential and to standardize the surface-charge of formulations, regardless of the strain and extract loading ratio. Indeed, pectin and alginate coated capsules showed zeta-potential values more negative than − 30 mV in all formulations, which technically ensures colloidal suspension stability and prevention of particle aggregation and flocculation [68]. Regarding the possible interaction of capsules with the intestinal mucosa, anionic polymers such as alginate and chitosan have proven to enhance the retention of capsules in the intestine by adhesion mechanisms to the intestinal mucosa, leading to increased intestinal absorption of encapsulated compunds [69,70,71]. Although the intestinal membrane is negatively charged, the carboxylic groups of pectin and alginate can form hydrogen bonds with oligosaccharide chains of intestinal mucins [72]. These interactions along with chain entanglement and van der Waal's interactions, are stronger that electrostatic repulsion, and result in high mucoadhesion of these biopolymers [73, 74]. Interestingly, a higher negative charge density in these polymers is related to the mucoadhesive strength and hence, the obtention of such negative zeta-potentials suggests that mucoadhesion and absorption of cannabinoids may be higher in pectin- and alginate-coated formulations compared to uncoated capsules [75, 76].
On the other hand, more differences were observed in the positive zeta-potentials of the capsules coated with chitosan. CI and CII 1:10 loaded capsules showed significantly higher zeta-potentials compared to CIII 1:10 loaded capsules, and CI 6:10 loaded capsules showed significantly higher values compared to CII and CIII 1:10 loaded capsules; whereas 3:10 loaded capsules showed comparable zeta-potentials regardless of the strain. Thus, CIII capsules showed slightly lower positive zeta-potentials compared to CI and CII capsules. However, the cannabis extract loading employed was the most critical factor that appeared to negatively incluence the zeta-potential value of chitosan-coated capsules. Increasing the loading ratio of cannabis extract led to significant decreases in the zeta-potential values of the chitosan-loaded capsules in all strains. Overall, zeta-potential values above + 30 mV were only achieved with 1:10 loaded CI and CII capsules, suggesting that the remaining chitosan-coated formulations would not result in stable aqueous dispersions, and mucoadhesive or mucopermeable properties might also be compromised [68]. The improved intestinal adhesion and permeability of chitosan (and other cationic polymers) coated capsules is the result of the electrostatic attraction between the positive charge of chitosan and the negative charge of intestinal membranes [77, 78]. A less positive surface charge of the capsules could compromise electrostatic attraction between capsules and intestinal membranes, and therefore, formulations with higher cannabis extract load (and less positive zeta-potentials) may exhibit less efficient absorption and a lower bioavailabilty of cannabinoids [75, 79].
In addition to particle-size and zeta-potential, the morphology of PLGA nanocapsules was evaluated by SEM analysis. Considering the minor influence of the nature of the employed cannabis strain in particle-size and zeta-potential, it was assumed that morphology of capsules would not vary significantly between capsules derived from different cannabis chemotypes. Hence, SEM images of all the formulations of one of the chemotypes (chemotype II) were taken and assumed to be representative for equivalent formulations (i.e. same extract load ratio and coating) of the other chemotypes (Figure S3). SEM images revealed certain heterogeneity in the morpohology of nanocapsules in most formulations. Although spherical or quasi-spherical shapes were predominant, elonged and irregular shapes were also observed. The polymeric coating used did not produce conclusive differencies in the shape of capsules. The extract loading ratio did not lead to clear differences in morphology neither, but appeared to be related to the agglomeration of nanocapsules. Regardless of the polymeric coating, a higher degree of agglomeration is observed with the increase of the loading ratio. This observation is in line with the fact that 10:10 loaded caspules could not be processed due to the formation of big agglomerates. This phenomenom led to the formation of some nanoparticle-networks in 6:10 loaded capsules, as it was also obvserved in the literature [51]. However, these agglomerations in dry nanocapsules seem to be reduced or to disappear when capsules are dispersed in water owing to the observed particle sizes and zeta-potential (and the measurement plots of both parameters). In regard to the surface, capsules with 1:10 extract load ratio seemed to have a smoother surface compared to capsules with higher loading ratios, but overall capsules presented rough and irregular surfaces.
Considering the observed tendencies and comparing the overall capsule morphologies with the spherical, regular and smooth surfaces obtained by Fraguas-Sanchez et. al, whose particle synthesis protocol was followed, it seems that the use of cannabis extracts instead of pure CBD, may influence negatively the morphology of capsules [33]. The observed angular and irregular morphology in some capsules could negatively affect their transport and membrane permeation features, and the lack of homogeneity may compromise the homogenous release of encapsulated compounds [80,81,82].
Although morphology of some nanocapsules could be improved, most formulations showed satisfactory particle sizes (confirmed by SEM imageing) and zeta-potenitals, and so, release and absorption of PLGA nanocapsules may still be suitable for a proper delivey of encapsulated cannabinoids [83, 84]. However, the use of lower cannabis extract loading ratios is recommended to assure the integrity of PLGA nanocapsules structure and the effectivity of the functionalization if chitosan is used. Similarly, if functionalization via anionic polymers is intended, the use of alginate is recommend over pectin, as pectin coating seemed to lead to a higher particle size in some cases.
In Vitro Gastro-intestinal Digestion Simulation: Release Profile and Bioaccessibility
In vitro gastro-intestinal digestion simulations were carried in order to get a preliminary idea of the release-profile and intestinal bioaccessibility of cannabinoids in the proposed PLGA nanoformulations. Moreover, these assays allow determining whether the employed cannabis strain, extract load ratio and coating has any effect on the release of cannabinoids of PLGA nanocapsules in gasto-intestinal media or not.
The release-profile of each formulation was determined based on the cumulative release of main cannabinoids (THC, THC and CBD, and CBD, in CI, CII and CIII strains respectively) during the in vitro gastro-intestinal digestion simulation. The raise of cumulative release during simulated digestion was graphically represented for each formulation (Figure S4). All formulations followed a sustained release profile of cannabinoids in both gastric and intestinal phases. In some cases though, the sustained release in the intestinal phase was preceded by a narrow burst release at the beginning of the intestinal phase. Despite the similarities in the release profiles, there are differences in release rate of main cannabinoids depending on the formulation. With the aim of having a deeper insight into the in vitro release profiles of the formulations studied in this work release kinetic models were built and compared. According to the literature, release mechanisms of drugs from PLGA nanocapsules are based on zero-order, first-order, Higuchi´s and Korsmeyer–Peppas´ Eqs. [85,86,87,88]. In this work, zero-order, first-order and Higuchi´s kinetic models were tested, which are common and basic kinetic models that work for most formulations, and give one kinetic constant per model, which eases the comparison among formulations [89]. The release data was split to obtain independent kinetics for the gastric and intestinal phases.
Higuchi´s kinetic model described best the cumulative release of cannabinoids from PLGA nanocapsules in most cases (81 out of 96). Although first-order kinetic model fitted better to the release of cannabinoids in the gastric phases of some formulations, Higuchi´s model had a similar fitting in most cases (see Table S4). This model fitted adequately to the release in both gastric and intestinal phases and the obtained coefficients of determination (r2) were above 0.9 in 83 out of 96 models (and above 0.95 in 65 out of 96 models). Those coefficients were only bellow 0.9 in most CIII 6:10 loaded formulations (r2 > 0.8) and in the gastric phases of CII60P, CII60A and CII60C capsules for THC (0.73 < r2 < 0.78). These results suggest that, in most of the synthetized nanocapsules, cannabinoids follow a fickian diffusion that fit to Higuchi´s kinetic model [89]. For those poor fitting curves, cannabinoids may follow a quasi-fickian or non-fickian diffusion that could better fit to Higuchi-derived models, such as Korsmeyer–Peppas´ model, as it has been previously reported [26, 86, 90]. In any case, the obtained release constants from Higuchi´s model (KH) in each phase (gastric and intestinal) were used to compare the release velocities among formulations.
The KH values of the gastric phase (Fig. 3A), show that the polymeric coating and the extract load ratio led to little or no significant differences among formulations. The KH values of formulations made with the same cannabis strain and extract load but different coating, did not differ statistically (p > 0.05), except for (i) 1:10 and 6:10 loaded CI capsules and 1:10 loaded CII capsules, where uncoated capsules showed higher KH values compared to coated ones, and (ii) 6:10 loaded CII capsules, where uncoated capsules showed lower KH values compared to pectin and chitosan coated ones. The extract load ratio led to significant differences in the release speed of CI capsules (1:10 and 3:10 loaded had higher KH compared to 6:10 loaded) and in CII uncoated and chitosan coated capsules (1:10 loaded had higher KH compared to 3:10 and 6:10 loaded ones), but did not lead to any significant difference in the rest of formulations. In contrast, the employed cannabis strain did lead to significant differences in most cases. Regardless of the extract load and polymeric coat, CIII capsules showed significantly higher KH values compared to CI and CII capsules, except for CI10Ø, CI30P, CI30A and CI30C capsules, which had comparable release speeds compared to their CIII analogous formulation. Additionally, CI capsules showed faster releases compared to CII capsules, excepting 6:10 loaded capsules were the contrary trend was observed.
Intestinal KH values (Fig. 3B), were statistically higher compared to the gastric KH values, probably due to the burst release observed in the beginning of the intestinal phase. There were a few excepcions though, such as CI 3:10 loaded, CII 6:10 loaded (only for THC) and CIII 3:10 loaded capsules where not show significant differences among digestive phases were observed, and CII (only for CBD) 6:10 and CIII 6:10 loaded capsules, which showed lower intestinal KH values.
Compared to the gastric phase, the used cannabis strain did not lead to significant differences in the release speed of cannabinoids in the intestinal phase. Although CII10Ø, CII10A, CII10C, CII30Ø and CII10C capsules had a significantly slower release compared to analogous CI and CIII formulations, the KH values of capsules with the same load and polymeric coat were comparable in the rest of cases for all the chemotypes. Similar to the gastric phase, the applied polymeric coat did not lead to significant differences in the intestinal release speed generally. In contrast, the extract load ratio, did lead to significant variations in all formulations. Regardless of the employed cannabis strain and polymeric coat, 1:10 loaded capsules showed significantly higher KH values compared to 3:10 loaded capsules in all cases (except for CI uncoated and pectin coated capsules); and 3:10 loaded capsules showed significantly higher KH values compared to 60% loaded capsules (except CII for pectin capsules and CIII uncoated and pectin capsules). This suggests that the release speed decreases as the extract load ratio increases.
The potential intestinal bioaccessibility of each formulation was estimated by substracting the fraction released at the end of the gastric phase to the cumulative release at the end of the in vitro gastro-intestinal simulation. The obtained in vitro bioaccessibility values ranged from 6 to 63%. The observed differences due to the variables (i.e., cannabis strain, extract load ratio and polymeric coat) of the formulations match with the tendencies observed in the KH values in the intestinal phase (Fig. 4). Analogously, nor the applied polymeric coat (except 6:10 loaded CII derived capsules, where uncoated capsules showed lower bioaccessibility values), neither the applied cannabis strain (except some cases where CII capsules showed lower bioaccessibility values) led to any significant changes in the bioaccessibility of THC and CBD. In contrast, lower bioaccessibility values were obtained for capsules loaded with higher cannabis extract to PLGA mass ratios. In fact, bioaccessibility values of 1:10 loaded capsules were in all cases (except in pectin coated CIII derived capsules) statistically higher (p-value < 0.05) than the values obtained for 6:10 loaded capsules.
The observations found for KH and bioaccessibility indicate that the cannabis extract loading has a significant effect on the release of cannabinoids from PLGA nanocapsules in gastro-intestinal media, rather than the employed cannabis strain or the functionalization via different polymers. In this context, it is worth mentioning the macroscopic differences observed when dry nanocapsules were handled for the in vitro bioaccessibility assays, which correspond to the structural differences observed in SEM images (Figure S3).
The appearance of capsules derived from different chemotypes or polymeric coatings were indistinguishable, whereas the extract load did led to noticeable changes. PLGA nanoparticles with 1:10 cannabis extract load were light brown color fine powders, but, as the cannabis extract load increased, nanocapsules capsules were darker and, more remarkably, got denser and stickier. In other words, as the cannabis extract load was increased the appearance of capsules got more similar to the employed cannabis extracts themselves, which were dark viscous and sticky oils as is the custom [91]. Similarly, dry PLGA nanocapsules loaded with higher ammounts of cannabis extracts, seemed to be stuck in agglomerates in the SEM images (Figure S3). These observations suggest that increasing cannabis extract load ratio may decrease porosity while increasing density. These structural changes can hinder the entrance of water and thus, the hydrolysis of PLGA, as well as blocking the diffusion of cannabinoids through the pores [92]. All these processes can decrease the release rate of entrapped compounds, which may explain the differences observed for KH and bioaccessibility values for capsules with different loading values. Similarly, the higher porosity in less loaded capsules, may explain the faster release of 1:10 loaded uncoated capsules compared to coated ones in the gastric phase, where PLGA hydrolysis is accelerated due to acidic catalysis [93]. Thus, in 1:10 loaded formulations the presence of an additional polymeric layer seems to decelerate the acidic catalyzed hydrolysis of PLGA, whereas in 3:10 and 6:10 loaded capsules the cannabis extract load to PLGA ratio seems to establish the release rate of cannabinoids.
Based on the results of the bioaccessibility assays, it can be concluded that loading PLGA nanocapsules with lower cannabis extract amounts may be more suitable in terms of effective intestinal dose, at least in in vitro conditions. The sticky and dense nature of the cannabis extract may hinder the release of cannabinoids through digestion. Due to this, cannabinoids remain entrapped in PLGA nanocapsules with higher extract loads, which results in a lower released dose in intestinal media, despite having a higher cannabinoid content.
The observed behavior of PLGA nanocapsules in simulated gastro-intestinal media and their physical characteristics suggest that capsules loaded with a 1:10 extract/PLGA ratio might be suitable for therapeutic applications. These capsules showed acceptable morphology, sufficient surface charge to provide colloidal stability and improved mucoadhesivity or mucopermeability, and suitable particle size in all cases, regardless of the polymeric coating applied or the nature of the cannabis strain. These formulations showed high intestinal bioaccesibilty and sustained release of cannabinoids, and their particle-size and zeta-potential were similar to the above-mentioned CB13 PLGA nanocapsules designed for oral administration, suggesting that their performance after oral ingestion could be similar [34,35,36,37].
Even though these similarities suggest that cannabinoid release, transport and absorption could be improved in comparison to other cannabinoid containing edibles, further characterization should be carried to confirm such hypotheses and elucidate real pharmacokinetics and bioavailabilty of the proposed formulations. In vivo experiments would be the most suitable approach to obtain such information [94]. However, cellular uptake of cannabinoids could be assessed using human colon adenocarcinoma (Caco-2) cell models, which is broadly accepted as a way to mimic intestinal absorption [95, 96]. This could be particularly interesting in order to assess the mucopermeablity and mucoadhesivity properties of the obtained PLGA nanocapsules, which could be substantially different among uncoated, alginate, pectin or chitosan coated nanocapsules, and could confirm the suitability of the size, shape and surface charge of nanocaspules [35, 97].
Conclusions
The value of full-spectrum formulations has gained visibility in recent years compared to pure THC, pure CBD or THC and CBD formulations. This fact, together with the suitability of PLGA-based systems for trapping cannabinoids and providing selective, sustained and effective cannabinoid release, has been the starting point for developing a series of PLGA-full-spectrum cannabis nanoformulations designed for oral intake.
For this purpose, full-spectrum cannabis extracts were obtained from natural sources, and have been encapsulated in PLGA nanovehicles following procedures to encapsulate pure cannabinoids. In order to cover a wide range of potential therapeutic uses and different formulation possibilities, we studied the use of three different cannabis strains and the use of three common polymeric coatings (alginate, pectin and chitosan) along with uncoated capsules. Owing to the lack of previous references using cannabis extracts for nanoencapsulation using PLGA, four cannabis extract mass to PLGA mass ratios were tested. All these variables would result in 48 different formulations. However, as the preparation of 12 of them was not feasible (the ones with 10:10 cannabis extract to PLGA load), 36 different nanocapsules were finally synthetized. Cannabinoid content, basic physical characterization and release of cannabinoids in simulated gastro-intestinal media were determined in order to get a preliminary idea of their suitability.
The cannabinoid content of THC and CBD was high in all formulations, and owing to the use of three different cannabis chemotypes, the proposed formulations could cover a wide range of therapeutic applications. Moreover, compared to other cannabinoid containing preparations, the proposed formulations maintained small amounts of CBC, CBG and other minor cannabinoids, which could result in the entourage effect often sought in cannabis based products. Related to this, the nature of the cannabis strain did not lead to major differencies in most of the studied properties, and so, it seems that the proposed formulations may be extended to additional strains if different cannabinoid profiles were sought.
Whereas the nature of the cannabis strain did not lead to significant differences, it was found that the amount used for the preparation of particles was critical. Indeed, when larger cannabis extract loads were used, the encapsulation efficiency, the functionalization with chitosan, the morphology and the release of cannabinoids in gastro-intestinal media was compromised. The viscous and dense nature of the cannabis extracts seemed to influence negatively the structure of capsules and obstruct significantly the release of cannabinoids, resulting in a substandard intestinal accessibility of free cannabinoids. Therefore, lower cannabis extract load based formulations are recommended for a better performance of PLGA nanocapsules.
The applied polymeric coatings on the other hand, did not lead to appreciable differences in most of the studied properties, but seemed suitable to improve mucoadhesiveness and mucopermeability compared to uncoated PLGA nanocapsules. Moreover, the assessed properties of capsules were generally comparable to those obtained in single cannabinoid loaded PLGA nanoformulations found in the literature, suggesting that the proposed formulations could have a similar in vivo performance.
Nonetheless, despite the potential benefits that the proposed formulations could have for oral cannabinoid administration, further assays related to their pharmacokinetics and bioavailability are needed in order to evaluate their suitability for medicinal purposes. In any case, the exposed results are useful as a starting point to develop new PLGA nanocapsules loaded with full-spectrum cannabis extracts.
Data Availability
Experimental data are not uploaded to any repository.
Abbreviations
- CI:
-
Chemotype I
- CII:
-
Chemotype II
- CIII:
-
Chemotype III;
- CBC:
-
Cannabichromene
- CBCA:
-
Cannabichromenic acid
- CBD:
-
Cannabidiol
- CBDA:
-
Cannabidiolic acid
- CBDV:
-
Cannabidivarin
- CBDVA:
-
Cannabidivarinic acid
- CBG:
-
Cannabigerol
- CBGA:
-
Cannabigerolic acid
- CBN:
-
Cannabinol
- CBNA:
-
Cannabinolic acid
- DAD:
-
Diode-array detector
- EE %:
-
Encapsulation-efficiency
- HPLC:
-
High-performance liquid chromatography
- IS:
-
Internal standard
- KH :
-
Higuchi´s model constant
- r2 :
-
Coefficient of determination
- SGF:
-
Simulated gastric fluid
- SIF:
-
Simulated intestinal fluid
- THC:
-
∆9-Tetrahydrocannabinol
- THCA:
-
∆9-Tetrahydrocannabinolic acid
- THCV:
-
∆9-Tetrahydrocannabivarin
- THCVA:
-
∆9-Tetrahydrocannabivarinic acid
- PDI:
-
Polydispersity index
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PVA:
-
Polyvinyl alcohol
References
Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M, et al. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol. 2018;227:300–15.
Russo EB, Grotenhermen F, editors. The Handbook of Cannabis Therapeutics: From Bench to Bedside. New York: Routledge; 2006;p 496
Di Marzo V, Piscitelli F. The Endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12(4):692–8.
Pascual D, Sánchez-Robles EM, García MM, Goicoechea C. Chronic pain and cannabinoids. Great expectations or a christmas carol. Biochem Pharmacol. 2018;157:33–42.
Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–64.
Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–50.
MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–9.
Walsh KB, McKinney AE, Holmes AE. Minor Cannabinoids: Biosynthesis, Molecular pharmacology and potential therapeutic uses. Frontiers in pharmacology [Internet]. 2021 [cited 2022 Dec 2];12. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2021.777804
Maayah ZH, Takahara S, Ferdaoussi M, Dyck JRB. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2020;1866(7):165771.
Lewis MA, Russo EB, Smith KM. Pharmacological foundations of cannabis chemovars. Planta Med. 2018;84(4):225–33.
Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23(10):2478.
Stella B, Baratta F, Della Pepa C, Arpicco S, Gastaldi D, Dosio F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs. 2021;81(13):1513–57.
Grotenhermen F. Clinical pharmacokinetics of cannabinoids. J Cannabis Therap. 2003;3(1):3–51.
Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–804.
Fairbairn JW, Liebmann JA, Rowan MG. The stability of cannabis and its preparations on storage. J Pharm Pharmacol. 1976;28(1):1–7.
Mechoulam R. Chemistry of Cannabis. In: Hoffmeister F, Stille G, editors. Psychotropic Agents: Part III: Alcohol and Psychotomimetics, Psychotropic Effects of Central Acting Drugs [Internet]. Berlin, Heidelberg: Springer; 1982 [cited 2022 Dec 16]. p. 119–34. (Handbook of Experimental Pharmacology). Available from: https://doi.org/10.1007/978-3-642-67770-0_7
Huestis MA. Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannibinol, Cannabidiol and Cannabinol. In: Pertwee RG, editor. Cannabinoids [Internet]. Berlin, Heidelberg: Springer; 2005 [cited 2024 Mar 28]. p. 657–90. Available from: https://doi.org/10.1007/3-540-26573-2_23
McClements DJ. Enhancing Efficacy, Performance, and reliability of cannabis edibles: Insights from lipid bioavailability studies. Annu Rev Food Sci Technol. 2020;11(1):45–70.
Light K, Karboune S. Emulsion, hydrogel and emulgel systems and novel applications in cannabinoid delivery: a review. Crit Rev Food Sci Nutr. 2022;62(29):8199–229.
Onaivi ES, Singh Chauhan BP, Sharma V. Challenges of cannabinoid delivery: how can nanomedicine help? Nanomedicine. 2020;15(21):2023–8.
Ahadian S, Finbloom JA, Mofidfar M, Diltemiz SE, Nasrollahi F, Davoodi E, et al. Micro and nanoscale technologies in oral drug delivery. Adv Drug Deliv Rev. 2020;157:37–62.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf, B. 2010;75(1):1–18.
Reddy TS, Zomer R, Mantri N. Nanoformulations as a strategy to overcome the delivery limitations of cannabinoids. Phytother Res. 2023;37(4):1526–38.
Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 2012;64:72–82.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J Control Release. 2012;161(2):505–22.
Hines DJ, Kaplan DL. Poly (lactic-co-glycolic acid) controlled release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. 2013;30(3):257–76.
Pardeshi SR, Nikam A, Chandak P, Mandale V, Naik JB, Giram PS. Recent advances in PLGA based nanocarriers for drug delivery system: a state of the art review. Int J Polym Mater Polym Biomater. 2021;72(1):49–78.
Abamor ES, Allahverdiyev A, Tosyali OA, Bagirova M, Acar T, Mustafaeva Z, et al. Evaluation of in vitro and in vivo immunostimulatory activities of poly (lactic-co-glycolic acid) nanoparticles loaded with soluble and autoclaved Leishmania infantum antigens: A novel vaccine candidate against visceral leishmaniasis. Asian Pac J Trop Med. 2019;12(8):353.
de Oliveira ALC, de Araújo Júnior RF de, Gomes de Carvalho T, B. Chan A, Schomann T, Tamburini F, et al. Effect of oxaliplatin-loaded poly (d,l-Lactide-co-Glycolic Acid) (PLGA) nanoparticles combined with retinoic acid and cholesterol on apoptosis, drug resistance, and metastasis factors of colorectal cancer. Pharmaceutics. 2020;12(2):193.
Fernandes C, Martins C, Fonseca A, Nunes R, Matos MJ, Silva R, et al. PEGylated PLGA Nanoparticles as a smart carrier to increase the cellular uptake of a coumarin-based monoamine oxidase B inhibitor. ACS Appl Mater Interfaces. 2018;10(46):39557–69.
Sun D, Li N, Zhang W, Yang E, Mou Z, Zhao Z, et al. Quercetin-loaded PLGA nanoparticles: a highly effective antibacterial agent in vitro and anti-infection application in vivo. J Nanopart Res. 2015;18(1):3.
Fraguas-Sánchez AI, Fernández-Carballido A, Simancas-Herbada R, Martin-Sabroso C, Torres-Suárez AI. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int J Pharm. 2020;574:118916.
Fraguas-Sánchez AI, Torres-Suárez AI, Cohen M, Delie F, Bastida-Ruiz D, Yart L, et al. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: In vitro and in ovo assessment. Pharmaceutics. 2020;12(5):439.
Berrocoso E, Rey-Brea R, Fernández-Arévalo M, Micó JA, Martín-Banderas L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomed Nanotechnol Biol Med. 2017;13(8):2623–32.
Durán-Lobato M, Muñoz-Rubio I, Holgado MÁ, Álvarez-Fuentes J, Fernández-Arévalo M, Martín-Banderas L. Enhanced cellular uptake and biodistribution of a synthetic cannabinoid loaded in surface-modified poly(lactic-co-glycolic acid) Nanoparticles. J Biomed Nanotechnol. 2014;10(6):1068–79.
Durán-Lobato M, Martín-Banderas L, Gonçalves LMD, Fernández-Arévalo M, Almeida AJ. Comparative study of chitosan- and PEG-coated lipid and PLGA nanoparticles as oral delivery systems for cannabinoids. J Nanopart Res. 2015;17(2):61.
Martín-Banderas L, Álvarez-Fuentes J, Durán-Lobato M, Prados J, Melguizo C, Fernández-Arévalo M, et al. Cannabinoid derivate-loaded PLGA nanocarriers for oral administration: formulation, characterization, and cytotoxicity studies. Int J Nanomedicine. 2012;7:5793–806.
Amin MK, Boateng J. Surface functionalization of PLGA nanoparticles for potential oral vaccine delivery targeting intestinal immune cells. Colloids Surf, B. 2023;222:113121.
El-Hammadi MM, Arias JL. Advanced engineering approaches in the development of PLGA-based nanomedicines. In: Aliofkhazraei M, editor. Handbook of Nanoparticles [Internet]. Cham: Springer International Publishing; 2015 [cited 2023 Aug 30]. p. 1–25. Available from: https://doi.org/10.1007/978-3-319-13188-7_45-1
Aldawsari HM, Alhakamy NA, Padder R, Husain M, Md S. Preparation and characterization of chitosan coated PLGA nanoparticles of resveratrol: Improved stability, Antioxidant and apoptotic activities in H1299 lung cancer cells. Coatings. 2020;10(5):439.
Azzazy HMES, Fahmy SA, Mahdy NK, Meselhy MR, Bakowsky U. Chitosan-coated PLGA nanoparticles loaded with Peganum harmala Alkaloids with promising antibacterial and wound healing activities. Nanomaterials. 2021;11(9):2438.
Dandamudi M, McLoughlin P, Behl G, Rani S, Coffey L, Chauhan A, et al. Chitosan-Coated PLGA Nanoparticles encapsulating triamcinolone acetonide as a potential candidate for sustained ocular drug delivery. Pharmaceutics. 2021;13(10):1590.
Fong SS, Foo YY, Saw WS, Leo BF, Teo YY, Chung I, et al. Chitosan-Coated-PLGA nanoparticles enhance the antitumor and antimigration activity of stattic – A STAT3 dimerization blocker. Int J Nanomed. 2022;17:137–50.
Martău GA, Mihai M, Vodnar DC. The Use of Chitosan, Alginate, and pectin in the biomedical and food sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers. 2019;11(11):1837.
Karp F, Satler FS, Busatto CA, Luna JA, Estenoz DA, Turino LN. Modulating drug release from poly(lactic-co-glycolic) acid microparticles by the addition of alginate and pectin. J Appl Polym Sci. 2021;138(17):50293.
Liu L, Fishman ML, Hicks KB, Kende M. Interaction of various pectin formulations with porcine colonic tissues. Biomaterials. 2005;26(29):5907–16.
Nastasi JR, Kontogiorgos V, Daygon VD, Fitzgerald MA. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci Technol. 2022;120:193–211.
Valdés A, Burgos N, Jiménez A, Garrigós MC. Natural pectin polysaccharides as edible coatings. Coatings. 2015;5(4):865–86.
Hariyadi DM, Islam N. Current status of alginate in drug delivery. Adv Pharmacol Pharm Sci. 2020;2020:e8886095.
Sanna V, Roggio AM, Siliani S, Piccinini M, Marceddu S, Mariani A, et al. Development of novel cationic chitosan-and anionic alginate–coated poly(d, l-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int J Nanomed. 2012;7:5501–16.
Wang Q, Jamal S, Detamore MS, Berkland C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J Biomed Mater Res, Part A. 2011;96A(3):520–7.
Lazzarotto Rebelatto ER, Rauber GS, Caon T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int J Pharm. 2023;635:122727.
Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, van Antwerp J, et al. Decarboxylation study of acidic cannabinoids: A novel approach using ultra-high-performance supercritical fluid chromatography/photodiode array-mass spectrometry. Cannabis Cannabinoid Res. 2016;1(1):262–71.
Song YX, Furtos A, Fuoco D, Boumghar Y, Patience GS. Meta-analysis and review of cannabinoids extraction and purification techniques. Canadian J Chem Eng. 2022;n/a(n/a):1–24.
Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J Nat Prod. 2016;79(2):324–31.
Holzer M, Vogel V, Mäntele W, Schwartz D, Haase W, Langer K. Physico-chemical characterisation of PLGA nanoparticles after freeze-drying and storage. Eur J Pharm Biopharm. 2009;72(2):428–37.
Bardsley K, Wimpenny I, Yang Y, Haj AJE. Fluorescent, online monitoring of PLGA degradation for regenerative medicine applications. RSC Adv. 2016;6(50):44364–70.
Della Porta G, Falco N, Giordano E, Reverchon E. PLGA microspheres by supercritical emulsion extraction: a study on insulin release in myoblast culture. J Biomater Sci Polym Ed. 2013;24(16):1831–47.
Pourasghar M, Koenneke A, Meiers P, Schneider M. Development of a fast and precise method for simultaneous quantification of the PLGA monomers lactic and glycolic acid by HPLC. J Pharm Anal. 2019;9(2):100–7.
Brodkorb A, Egger L, Alminger M, Alvito P, Assunção R, Ballance S, et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc. 2019;14(4):991–1014.
Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, et al. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014;5(6):1113–24.
Bhaskar A, Bell A, Boivin M, Briques W, Brown M, Clarke H, et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: results of a modified Delphi process. J Cannabis Res. 2021;3(1):22.
Marangoni IP, Marangoni AG. Cannabis edibles: dosing, encapsulation, and stability considerations. Curr Opin Food Sci. 2019;28:1–6.
Carbone M, Castelluccio F, Daniele A, Sutton A, Ligresti A, Di Marzo V, et al. Chemical characterisation of oxidative degradation products of Δ9-THC. Tetrahedron. 2010;66(49):9497–501.
Meija J, McRae G, Miles CO, Melanson JE. Thermal stability of cannabinoids in dried cannabis: a kinetic study. Anal Bioanal Chem. 2022;414(1):377–84.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
Dynamic Light Scattering (DLS) - Definition & Terms | Malvern Panalytical [Internet]. [cited 2023 Sep 5]. Available from: https://www.malvernpanalytical.com/es/learn/knowledge-center/whitepapers/wp111214dlstermsdefined
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Chapter 10 - Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In: Tekade RK, editor. Basic Fundamentals of Drug Delivery [Internet]. Academic Press; 2019 [cited 2023 Sep 5]. p. 369–400. (Advances in Pharmaceutical Product Development and Research). Available from: https://www.sciencedirect.com/science/article/pii/B9780128179093000108
Chickering DE, Mathiowitz E. Bioadhesive microspheres: I. A novel electrobalance-based method to study adhesive interactions between individual microspheres and intestinal mucosa. J Control Release. 1995;34(3):251–62.
Ch’Ng HS, Park H, Kelly P, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery II: Synthesis and evaluation of some swelling, Water-insoluble bioadhesive polymers. J Pharm Sci. 1985;74(4):399–405.
Zandanel C, Ponchel G, Noiray M, Vauthier C. Nanoparticles facing the gut barrier: Retention or mucosal absorption? Mechanisms and dependency to nanoparticle characteristics. Int J Pharm. 2021;609:121147.
Khutoryanskiy VV. Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci. 2011;11(6):748–64.
Laffleur F, Netsomboon K, Bernkop-Schnürch A, Westmeier D, Stauber RH, Docter D. Comprehensive mucoadhesive study of anionic polymers and their derivate. Eur Polymer J. 2017;93:314–22.
Yermak IM, Davydova VN. Volod’ko AV. Mucoadhesive Marine Polysaccharides Mar Drugs. 2022;20(8):522.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems - a review (Part 1). Trop J Pharm Res. 2013;12(2):255–64.
Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12(9–10):635–41.
Farshbaf M, Davaran S, Zarebkohan A, Annabi N, Akbarzadeh A, Salehi R. Significant role of cationic polymers in drug delivery systems. Artif Cells, Nanomedicine, Biotechnol. 2018;46(8):1872–91.
Schulz JD, Gauthier MA, Leroux JC. Improving oral drug bioavailability with polycations? Eur J Pharm Biopharm. 2015;1(97):427–37.
Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52(2):117–26.
Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121(1):3–9.
Venditti I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J King Saud University - Sci. 2019;31(3):398–411.
Zhang W, Taheri-Ledari R, Ganjali F, Mirmohammadi SS, Qazi FS, Saeidirad M, et al. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: a review. RSC Adv. 2022;13(1):80–114.
Modena MM, Rühle B, Burg TP, Wuttke S. Nanoparticle characterization: What to measure? Adv Mater. 2019;31(32):1901556.
Sandri G, Bonferoni MC, Ferrari F, Rossi S, Caramella CM. The Role of Particle Size in Drug Release and Absorption. In: Merkus HG, Meesters GMH, editors. Particulate Products: Tailoring Properties for Optimal Performance [Internet]. Cham: Springer International Publishing; 2014 [cited 2024 Mar 30]. p. 323–41. Available from: https://doi.org/10.1007/978-3-319-00714-4_11
Bhosale U, Devi K, Choudhary S. Development and in vitro-in vivo evaluation of oral drug delivery system of acyclovir loaded PLGA nanoparticles. Int J Drug Del. 2013;5(3):331–43.
Bohrey S, Chourasiya V, Pandey A. Polymeric nanoparticles containing diazepam: preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Convergence. 2016;3(1):3.
Ryu S, Park S, Lee HY, Lee H, Cho CW, Baek JS. Biodegradable nanoparticles-loaded PLGA microcapsule for the enhanced encapsulation efficiency and controlled release of hydrophilic drug. Int J Mol Sci. 2021;22(6):2792.
Zakeri-Milani P, Loveymi BD, Jelvehgari M, Valizadeh H. The characteristics and improved intestinal permeability of vancomycin PLGA-nanoparticles as colloidal drug delivery system. Colloids Surf, B. 2013;1(103):174–81.
Bruschi ML, editor. 5 - Mathematical models of drug release. In: Strategies to Modify the Drug Release from Pharmaceutical Systems [Internet]. Woodhead Publishing; 2015 [cited 2022 Dec 19]. p. 63–86. Available from: https://www.sciencedirect.com/science/article/pii/B9780081000922000059
Ünal S, Doğan O, Aktaş Y. Orally administered docetaxel-loaded chitosan-decorated cationic PLGA nanoparticles for intestinal tumors: formulation, comprehensive in vitro characterization, and release kinetics. Beilstein J Nanotechnol. 2022;13(1):1393–407.
Romano L, Hazekamp A. Cannabis Oil: chemical evaluation of an upcoming cannabis-based medicine. In 2013 [cited 2023 Oct 12]. Available from: https://www.semanticscholar.org/paper/Cannabis-Oil%3A-chemical-evaluation-of-an-upcoming-Romano-Hazekamp/124a1b6d7c6c29aa8c10248e988c95b708e094dd
Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int J Pharm. 2011;415(1):34–52.
Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J Control Release. 2007;122(3):338–44.
Dima C, Assadpour E, Dima S, Jafari SM. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Comprehensive Reviews in Food Science and Food Safety. 2020;19(6):2862–84.
Angelis ID, Turco L. Caco-2 Cells as a model for intestinal absorption. Curr Prot Toxicol. 2011;47(1):20.6.1–20.6.15.
van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 2005;1(2):175–85.
Morelli L, Gimondi S, Sevieri M, Salvioni L, Guizzetti M, Colzani B, et al. Monitoring the fate of orally administered PLGA nanoformulation for local delivery of therapeutic drugs. Pharmaceutics. 2019;11(12):658.
Acknowledgements
A. Villate and M. San Nicolas are grateful to the IBeA consolidated research group and to the University of the Basque Country for their predoctoral fellowships.
G. Barreto is researcher of CIC CONICET – Argentine.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Basque Government through the financial support as consolidated group of the Basque Research System (IT1213-19 and IT1446-22).
Author information
Authors and Affiliations
Contributions
Conceptualization, G.B., O.A.-O., M.O. and A.U.; methodology, M.O. and A.U.; formal analysis, A.V., G.B., M.S.N., O.A.-O., M.O. and A.U.; investigation, A.V., G.B., and M.S.N.; resources, O.A.-O. and A.U.; data curation, M.O. and A.U.; writing—original draft preparation, A.V.; writing—review and editing, A.V., G.B, M.O. and A.U.; visualization, A.V.; supervision, O.A.-O., M.O. and A.U.; project administration, A.U.; funding acquisition, A.U. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Villate, A., Barreto, G.P., Nicolás, M.S. et al. Development, Characterization and In Vitro Gastrointestinal Release of PLGA Nanoparticles Loaded with Full-Spectrum Cannabis Extracts. AAPS PharmSciTech 25, 120 (2024). https://doi.org/10.1208/s12249-024-02836-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02836-4