Abstract
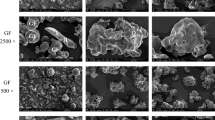
The purpose of this study is to develop modified particles with different structures to improve the flowability and compactibility of Liuwei Dihuang (LWDH) powder using co-spray drying technology, and to investigate the preparation mechanism of modified particles and their modified direct compaction (DC) properties. Moreover, tablets with high drug loading contents were also prepared. Particles were designed using polyvinylpyrrolidone (PVP K30) and hydroxypropyl methylcellulose (HPMC E3) as shell materials, and sodium bicarbonate (NaHCO3) and ammonium bicarbonate (NH4HCO3) as pore-forming agents. The porous particles (Ps), core-shell particles (CPs), and porous core-shell particles (PCPs) were prepared by co-spray drying technology. The key DC properties and texture properties of all the particles were measured and compared. The properties of co-spray drying liquid were also determined and analyzed. According to the results, Ps showed the least improvement in DC properties, followed by CPs, and PCPs showed a significant improvement. The modifier, because of its low surface tension, was wrapped in the outer layer to form a shell, and the pore-forming agent was thermally decomposed to produce pores, forming core-shell, porous, and porous core-shell composite structures. The smooth surface of the shell structure enhances fluidity, while the porous structure allows for greater compaction space, thereby improving DC properties during the compaction process.
Graphical Abstract










Similar content being viewed by others
Data Availability
The data generated and analyzed in the study are included in this article. Any additional supportive data are available on request from the corresponding author, upon reasonable request.
References
Adeoye O, Alebiowu G. Flow, packing and compaction properties of novel coprocessed multifunctional directly compressible excipients prepared from tapioca starch and mannitol. Pharm Dev Technol. 2014;19(8):901–10. https://doi.org/10.3109/10837450.2013.840843.
Kottlan A, Zirkl A, Geistlinger J, Machado Charry E, Glasser BJ, Khinast JG. Single-tablet-scale direct-compression: an on-demand manufacturing route for personalized tablets. Int J Pharm. 2023;643: 123274. https://doi.org/10.1016/j.ijpharm.2023.123274.
Li Z, Peng W-H, Liu W-J, Yang L-Y, Naeem A, Feng Y, et al. Advances in numerical simulation of unit operations for tablet preparation. Int J Pharm. 2023;634:122638. https://doi.org/10.1016/j.ijpharm.2023.122638.
Li YN, Wu ZF, Wan N, Li YH, Li HT, Yang M. Current situations and problem analysis of influencing factors of traditional Chinese medicine tablets on forming quality. Zhongguo Zhong Yao Za Zhi. 2018;43(8):1547–53. https://doi.org/10.19540/j.cnki.cjcmm.20180117.005.
Liu H, Yang S, Yang M. Modernization of traditional Chinese medicine manufacturing: research and application of key technologies in industrialization of solid preparations. Mod Chin Med. 2020;22:155–61.
Nakamura H, Baba T, Ohsaki S, Watano S, Takehara K, Higuchi T. Numerical simulation of wet granulation using the DEM–PBM coupling method with a deterministically calculated agglomeration kernel. Chem Eng J. 2022;450:138298. https://doi.org/10.1016/j.cej.2022.138298.
Thapa P, Tripathi J, Jeong SH. Recent trends and future perspective of pharmaceutical wet granulation for better process understanding and product development. Powder Technol. 2019;344:864–82. https://doi.org/10.1016/j.powtec.2018.12.080.
Zhang Y, Li J, Gao Y, Wu F, Hong Y, Shen L, et al. Improvements on multiple direct compaction properties of three powders prepared from Puerariae lobatae radix using surface and texture modification: comparison of microcrystalline cellulose and two nano-silicas. Int J Pharm. 2022;622:121837. https://doi.org/10.1016/j.ijpharm.2022.121837.
Zhang Y, Li Y, Wu F, Hong Y, Shen L, Lin X, et al. Texture and surface feature-mediated striking improvements on multiple direct compaction properties of Zingiberis rhizoma extracted powder by coprocessing with nano-silica. Int J Pharm. 2021;603:120703. https://doi.org/10.1016/j.ijpharm.2021.120703.
Bogdan C, Hales D, Cornilă A, Casian T, Iovanov R, Tomuță I, et al. Texture analysis – a versatile tool for pharmaceutical evaluation of solid oral dosage forms. Int J Pharm. 2023;638:122916. https://doi.org/10.1016/j.ijpharm.2023.122916.
Hadinoto K, Tran T-T, Chua A, Cheow WS. Comparing environmental impacts of direct compaction versus wet granulation tableting methods for drugs with poor flowability by life cycle assessment. Chem Eng Res Des. 2022;183:439–51. https://doi.org/10.1016/j.cherd.2022.05.029.
Li Z, Wu F, Zhao L, Lin X, Shen L, Feng Y. Evaluation of fundamental and functional properties of natural plant product powders for direct compaction based on multivariate statistical analysis. Adv powder technol. 2018;29(11):2881–94.
Ficzere M, Mészáros LA, Madarász L, Novák M, Nagy ZK, Galata DL. Indirect monitoring of ultralow dose API content in continuous wet granulation and tableting by machine vision. Int J Pharm. 2021;607:121008. https://doi.org/10.1016/j.ijpharm.2021.121008.
Gao Y, Li J, Zhao L, Hong Y, Shen L, Wang Y, et al. Distribution pattern and surface nature-mediated differential effects of hydrophilic and hydrophobic nano-silica on key direct compaction properties of Citri Reticulatae Pericarpium powder by co-processing. Powder Technol. 2022;404:117442. https://doi.org/10.1016/j.powtec.2022.117442.
Li Z, Xian J, Wu F, Lin X, Shen L, Feng Y. Development of TCM-based composite particles for direct compaction by particle design. Powder Technol. 338 481–492. https://doi.org/10.1016/j.powtec.2018.07.014
Vanhoorne V, Peeters E, Van Snick B, Remon JP, Vervaet C. Crystal coating via spray drying to improve powder tabletability. Eur J Pharm Biopharm. 2014;88(3):939–44. https://doi.org/10.1016/j.ejpb.2014.10.018.
De Pauw E, Vervaet C, Vanhoorne V. Formation of delta-mannitol by co-spray drying: enhancing the tabletability of paracetamol/mannitol formulations. J Drug Deliv Sci Tec. 2022;77:103907. https://doi.org/10.1016/j.jddst.2022.103907.
Remadevi R, Av Morton D, Hapgood K, Rashida N, Rajkhowa R. Improving the dynamic properties of silk particles by co-spray drying with L-leucine. Adv Powder Technol. 2022;33(5):103556. https://doi.org/10.1016/j.apt.2022.103556.
Gallo L, Piña J, Bucalá V, Allemandi D, Ramírez-Rigo MV. Development of a modified-release hydrophilic matrix system of a plant extract based on co-spray-dried powders. Powder Technology. 2013;241:252–62. https://doi.org/10.1016/j.powtec.2013.03.011.
Li Z, Zhu L, Chen FC, Guan YM, Chen LH, Zhang JW, et al. The preparation, characterization, and application of porous core-shell composite particles produced with laboratory-scale spray dryer. Drug Dev Ind Pharm. 2023;49(2):217–31. https://doi.org/10.1080/03639045.2023.2197070.
Chen C, Liu M, Lii S, Gao C, Chen J. In vitro degradation and drug-release properties of water-soluble chitosan cross-linked oxidized sodium alginate core-shell microgels. J Biomater Sci Polym Ed. 2012;23(16):2007–24. https://doi.org/10.1163/092050611X601720.
Zhou M, Shen L, Lin X, Hong Y, Feng Y. Design and pharmaceutical applications of porous particles. RSC advances. 2017;7(63):39490–501. https://doi.org/10.1039/C7RA06829H.
Nadaf S, Jadhav A, Killedar S. Mung bean (Vigna radiata) porous starch for solubility and dissolution enhancement of poorly soluble drug by solid dispersion. Int J Biol Macromol. 2021;167:345–57. https://doi.org/10.1016/j.ijbiomac.2020.11.172.
Han X, Ma P, Shen M, Wen H, Xie J. Modified porous starches loading curcumin and improving the free radical scavenging ability and release properties of curcumin. Food Res Int. 2023;168:112770. https://doi.org/10.1016/j.foodres.2023.112770.
Blaesi AH, Saka N. Microstructural effects in drug release by solid and cellular polymeric dosage forms: a comparative study. Mater Sci Eng C Mater Biol Appl. 2017;80:715–27. https://doi.org/10.1016/j.msec.2017.05.080.
Li Z, Zhou M, Wu F, Shen L, Lin X, Feng Y. Direct compaction properties of Zingiberis rhizoma extracted powders coated with various shell materials: improvements and mechanism analysis. Int J Pharm. 2019;564:10–21. https://doi.org/10.1016/j.ijpharm.2019.04.021.
Smith WR, Wang QX. The role of surface tension on the growth of bubbles by rectified diffusion. Ultrason Sonochem. 2023;98:106473. https://doi.org/10.1016/j.ultsonch.2023.106473.
Fadlalla D, Rosettani J, Ghazanfari Holagh S, Ahmed WH. Airlift pumps characteristics for shear-thinning non-Newtonian fluids: an experimental investigation on liquid viscosity impact. Exp Therm Fluid Sci. 2023;149:110994. https://doi.org/10.1016/j.expthermflusci.2023.110994.
Li Z, Jiang X, Huang H, Liu A, Liu H, Abid N, et al. Chitosan/zein films incorporated with essential oil nanoparticles and nanoemulsions: similarities and differences. Int J Biol Macromol. 2022;208:983–94. https://doi.org/10.1016/j.ijbiomac.2022.03.200.
Singh SY, Verma R, Kumar L. Porous oral drug delivery system: tablets. Pharm Chem J. 2018;52(6):553–61. https://doi.org/10.1007/s11094-018-1859-5.
Wang H, Wang E, Liu Z, Gao D, Yuan R, Sun L, et al. A novel carbon nanotubes reinforced superhydrophobic and superoleophilic polyurethane sponge for selective oil–water separation through a chemical fabrication. J Mater Chem A. 2015;3(1):266–73. https://doi.org/10.1039/c4ta03945a.
Li J, Li Z, Ruan H, Gao Y, Hong Y, Shen L, et al. Improved direct compression properties of Gardeniae fructus water extract powders via fluid bed-mediated surface engineering. Pharm Dev Technol. 2022;27(6):725–39. https://doi.org/10.1080/10837450.2022.2109671.
Tsolaki E, Stocker MW, Healy AM, Ferguson S. Formulation of ionic liquid APIs via spray drying processes to enable conversion into single and two-phase solid forms. Int J Pharm. 2021;603:120669. https://doi.org/10.1016/j.ijpharm.2021.120669.
Paul S, Taylor LJ, Murphy B, Krzyzaniak JF, Dawson N, Mullarney MP, et al. Powder properties and compaction parameters that influence punch sticking propensity of pharmaceuticals. Int J Pharm. 2017;521(1–2):374–83. https://doi.org/10.1016/j.ijpharm.2017.02.053.
Guimarães AA, Klein TS, Medronho RdA. Fish-hook effect in granulometric efficiency curves of hydrocyclones: a misuse of laser diffraction particle size analysers. Powder Technol. 2020;374:185-189. https://doi.org/10.1016/j.powtec.2020.06.091
Singh SY, Salwa, Shirodkar RK, Verma R, Kumar L. Enhancement in dissolution rate of atorvastatin trihydrate calcium by formulating its porous tablet using sublimation technique. J Pharm Innov. 2019;15(4):498–520. https://doi.org/10.1007/s12247-019-09397-1.
Deng T, Garg V, Salehi H, Bradley MSA. An experimental study on free-surface rolling segregation and correlations with angle of repose and particle sphericity. Powder Technol. 2021;379:307–20. https://doi.org/10.1016/j.powtec.2020.10.077.
Sun W-J, Chen H, Aburub A, Sun CC. A platform direct compression formulation for low dose sustained-release tablets enabled by a dual particle engineering approach. Powder Technol. 2019;342:856–63. https://doi.org/10.1016/j.powtec.2018.10.054.
van der Haven DLH, Ørtoft FH, Naelapää K, Fragkopoulos IS, Elliott JA. Predictive modelling of powder compaction for binary mixtures using the finite element method. Powder Technol. 2022;403:117381. https://doi.org/10.1016/j.powtec.2022.117381.
Xia X, Li F, Ran H, Zhao J, Lei X, Lei L, et al. Effect of jujube kernel powder addition on moisture absorption performance, color stability, texture properties and agglomeration characteristics of jujube powder. Lwt. 2023;174:114452. https://doi.org/10.1016/j.lwt.2023.114452.
Li J, Wang Z, Xiu H, Zhao X, Ma F, Liu L, et al. Correlation between the powder characteristics and particle morphology of microcrystalline cellulose (MCC) and its tablet application performance. Powder Technol. 2022;399:117194. https://doi.org/10.1016/j.powtec.2022.117194.
Sinka IC, Motazedian F, Cocks ACF, Pitt KG. The effect of processing parameters on pharmaceutical tablet properties. Powder Technol. 2009;189(2):276–84. https://doi.org/10.1016/j.powtec.2008.04.020.
Almukainzi M, Alobaid R, Aldosary M, Aldalbahi Y, Bashiri M. Investigation of the effects of different beverages on the disintegration time of over-the-counter medications in Saudi Arabia. Saudi Pharm J. 2021;29(7):699–705. https://doi.org/10.1016/j.jsps.2021.04.032.
Kaerger JS, Edge S, Price R. Influence of particle size and shape on flowability and compactibility of binary mixtures of paracetamol and microcrystalline cellulose. Eur J Pharm Sci. 2004;22(2–3):173–9. https://doi.org/10.1016/j.ejps.2004.03.005.
Zhou M, Wang Y, Wu F, Shen L, Lin X, Feng Y. Development on porous particles of Pueraria lobatae radix for improving its compactibility and dissolution. RSC Adv. 2018;8(43):24250–60. https://doi.org/10.1039/c8ra04125c.
Chendo C, Pinto JF, Paisana MC. Comprehensive powder flow characterization with reduced testing. Int J Pharm. 2023;642:123107. https://doi.org/10.1016/j.ijpharm.2023.123107.
Heng PW, Chan LW, Liew CV, Chee SN, Soh JL, Ooi SM. Roller compaction of crude plant material: influence of process variables, polyvinylpyrrolidone, and co-milling. Pharm Dev Technol. 2004;9(2):135–44. https://doi.org/10.1081/pdt-120027425.
Crouter A, Briens L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech. 2014;15(1):65–74. https://doi.org/10.1208/s12249-013-0036-0.
Agrawal A, Dudhedia M, Deng W, Shepard K, Zhong L, Povilaitis E, et al. Development of tablet formulation of amorphous solid dispersions prepared by hot melt extrusion using quality by design approach. AAPS PharmSciTech. 2016;17(1):214–32. https://doi.org/10.1208/s12249-015-0472-0.
Yuan J, Shi L, Sun W-J, Chen J, Zhou Q, Sun CC. Enabling direct compression of formulated Danshen powder by surface engineering. Powder Technol. 2013;241:211–8. https://doi.org/10.1016/j.powtec.2013.03.010.
Mašková E, Kubová K, Raimi-Abraham BT, Vllasaliu D, Vohlídalová E, Turánek J, et al. Hypromellose – a traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J Control Release. 2020;324:695–727. https://doi.org/10.1016/j.jconrel.2020.05.045.
Chen F-C, Liu W-J, Zhu W-F, Yang L-Y, Zhang J-W, Feng Y, et al. Surface modifiers on composite particles for direct compaction. Pharmaceutics. 2022;14(10). https://doi.org/10.3390/pharmaceutics14102217
Abu-hardan M, Hill SE. Handling properties of cereal materials in the presence of moisture and oil. Powder Technol. 2010;198(1):16–24. https://doi.org/10.1016/j.powtec.2009.10.002.
Alhajj N, O’Reilly NJ, Cathcart H. Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021;384:313–31. https://doi.org/10.1016/j.powtec.2021.02.031.
Carrigy N, Vehring R. Engineering stable spray-dried biologic powder for inhalation. Pharm inhalation aerosol technol. 2019:291-326.
Vehring R, Foss WR, Lechuga-Ballesteros D. Particle formation in spray drying. J Aerosol Sci. 2007;38(7):728–46. https://doi.org/10.1016/j.jaerosci.2007.04.005.
Rajasekar K, Raja B. Heat and mass transfer characteristics during spray drying of Na2Fe0.6Mn0.4PO4F/C cathode material for Na-ion batteries. Appl Therm Eng. 2023;221:119838. https://doi.org/10.1016/j.applthermaleng.2022.119838
Zhang X, Zhou Y, Wang G, Zhao Z, Jiang Z, Cui Y, et al. Co-spray-dried poly-L-lysine with L-leucine as dry powder inhalations for the treatment of pulmonary infection: moisture-resistance and desirable aerosolization performance. Int J Pharm. 2022;624:122011. https://doi.org/10.1016/j.ijpharm.2022.122011.
Kajihara R, Noguchi S, Iwao Y, Yasuda Y, Segawa M, Itai S. Structural investigation of spherical hollow excipient Mannit Q by X-ray microtomography. Int J Pharm. 2015;495(1):140–3. https://doi.org/10.1016/j.ijpharm.2015.08.097.
Sorenson EC, Woolley EM. Thermodynamics of proton dissociation from aqueous bicarbonate: apparent molar volumes and apparent molar heat capacities of potassium carbonate and potassium bicarbonate at T=(278.15 to 393.15) K and at the pressure 0.35 MPa. J Chem Thermody. 2004;36(4):289–98. https://doi.org/10.1016/j.jct.2003.12.008.
Berg RL, Vanderzee CE. Thermodynamics of carbon dioxide and: (a) the standard enthalpies of solution of Na2CO3(s), NaHCO3(s), and CO2(g) in water at 298.15 K; (b) the standard enthalpies of formation, standard Gibbs energies of formation, and standard entropies of CO2(aq), HCO3−(aq), CO32−(aq), NaHCO3(s), Na2CO3(s), Na2CO3·H2O(s), and Na2CO3·10H2O(s). J Chem Thermodyn. 1978;10(12):1113–36.
Guo J, Wang J, Wang W, Bai Z, Zhang Z, Zhang Y, et al. The fabrication of 3D porous PDMS sponge for oil and organic solvent absorption. Environ Prog Sustain. 2019;38(s1):S86–92. https://doi.org/10.1002/ep.12924.
Frykstrand S, Forsgren J, Mihranyan A, Strømme M. On the pore forming mechanism of Upsalite, a micro- and mesoporous magnesium carbonate. Micropor Mesopor Mat. 2014;190:99–104. https://doi.org/10.1016/j.micromeso.2013.12.011.
Al-Khattawi A, Koner J, Rue P, Kirby D, Perrie Y, Rajabi-Siahboomi A, et al. A pragmatic approach for engineering porous mannitol and mechanistic evaluation of particle performance. Eur J Pharm Biopharm. 2015;94:1–10. https://doi.org/10.1016/j.ejpb.2015.04.011.
Acknowledgements
We are grateful to Jiang Zhong Pharmaceutical Co., Ltd. for their support in the extraction and concentration of Chinese medicinal materials. We are also grateful to the Key Laboratory of Jiangxi University of Chinese Medicine for supporting the determination of key properties of the powder by DC.
Funding
This research was funded by the National Natural Science Foundation of China (82003953, 82360777), China Postdoctoral Science Foundation (2019M662278), National Natural Science Foundation of Jiangxi Province (20202BAB216039, 20232ACB216015, 20212BAB216009, and 20232BAB206166), 2020–2022 Young Talents Support Project of Chinese Society of Chinese Medicine (2020-QNRC2-07), Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (CXTD-22004), Doctoral Research Start-up Fund Project of Jiangxi University of Chinese Medicine (2021BSZR015, 2022BSZR003), Training Plan for Academic and Technical Leaders of Major Disciplines in Jiangxi Province (20204BCJL22048), and 2023 Innovation and Entrepreneurship Training Program of Jiangxi University of Chinese Medicine (X202310412208).
Author information
Authors and Affiliations
Contributions
ZL conceived/designed the study. ZL, WP, and LZ carried out the experiments and data collection. LC, AN, and YF carried out the analysis and interpretation of data. WP, LZ, WL, and LY drafted the manuscript. ZL, WZ, and LM revised and critically reviewed the work for important intellectual content and gave final approval of the version for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Peng, W., Zhu, L. et al. Study on Improving the Performance of Traditional Medicine Extracts with High Drug Loading Based on Co-spray Drying Technology. AAPS PharmSciTech 24, 247 (2023). https://doi.org/10.1208/s12249-023-02703-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02703-8