Abstract
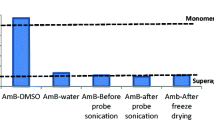
Amphotericin B (AmB) is a membrane-acting antibiotic used for the treatment of fungal and protozoal infections. AmB exists in various molecular forms, i.e., monomeric, super-aggregated, and oligomeric forms, where oligomeric forms are highly toxic because of their relative affinity toward cholesterol present over human cell membrane. Hence, the objective of our research work was to study the aggregation state of AmB in two different nanoformulations, i.e., solid lipid nanoparticles (SLNs) and zein-based nanoparticles (PNPs), with the aim of enhancing the fraction of less toxic form of AmB, and a comparative study was performed. The zein and glyceryl monostearate can intercalate the polyenic domain of AmB and thereby hinder the hydrophobic attractions between the AmB molecules, which allows their existence in monomeric forms. The particle size of AmB-SLNs and AmB-PNPs were 378.90 ± 9.50 nm and 184.90 ± 6.00 nm, while zeta potential was −34.97 ± 0.51 mV and +28.93 ± 2.29 mV, respectively. In vitro release studies showed more controlled release of AmB from PNPs (52.48 ± 1.07%) as compared to SLNs (86.33 ± 0.93%). The predominant aggregation state of AmB in both formulations was determined by UV–visible and circular dichroism spectrophotometry, where a higher degree of monomerization of AmB was reported in AmB-SLNs as compared to AmB-PNPs. Toxicity of the nanoformulations was evaluated through hemolysis test, where the results suggested that AmB-SLNs and AmB-PNPs were less hemolytic as compared to pure AmB. The nanoformulations demonstrated the predominant monomeric form of AmB, which may offer higher selectivity index toward microbial membrane.
Graphical Abstract















Similar content being viewed by others
Data Availability
The authors confirm that all the data supporting the findings of this study are presented within the article. Also, raw data that support the findings of this research may provided upon reasonable request.
Abbreviations
- % CDR:
-
Cumulative drug release
- AmB:
-
Amphotericin B
- CD:
-
Circular dichroism spectroscopy
- DL:
-
Drug loading
- DLS:
-
Dynamic light scattering
- DSC:
-
Differential scanning calorimetry
- EE:
-
Entrapment efficiency
- FTIR:
-
Fourier-transform infrared spectroscopy
- GMS:
-
Glyceryl monostearate
- PBS:
-
Phosphate buffer saline
- PDI:
-
Polydispersity index
- PNPs:
-
Polymeric nanoparticles
- RDI:
-
Redispersibility index
- RH:
-
Relative humidity
- SD:
-
Standard deviation
- SEM:
-
Scanning electron microscopy
- SLNs:
-
Solid lipid nanoparticles
- SL:
-
Soy lecithin
- TDW:
-
Triple-distilled water
References
Jain K, Jain NK. Novel therapeutic strategies for treatment of visceral leishmaniasis. Drug Discov Today. 2013;18:1272–81.
Serrano DR, Lalatsa A. Oral amphotericin B: the journey from bench to market. J Drug Deliv Sci Technol. 2017;42:75–83.
Jain K, Verma AK, Mishra PR, Jain NK. Surface-engineered dendrimeric nanoconjugates for macrophage-targeted delivery of amphotericin B: formulation development and in vitro and in vivo evaluation. Antimicrob Agents Chemother. 2015;59(5):2479–87.
Jain V, Jain K. Molecular targets and pathways for the treatment of visceral leishmaniasis. Drug Discov Today. 2018;23(1):161–70.
Das S, Devarajan PV. Enhancing safety and efficacy by altering the toxic aggregated state of amphotericin B in lipidic nanoformulations. Mol Pharm. 2020;17(6):2186–95.
Zia Q, Azhar A, Amjad Kamal M, Aliev G, Owais M, Md Ashraf G. Super aggregated form of Amphotericin B: a novel way to increase its therapeutic index. Curr Pharm Des. 2016;22(7):792–803.
Tevyashova AN, Olsufyeva EN, Solovieva SE, Printsevskaya SS, Reznikova MI, Trenin AS, et al. Structure-antifungal activity relationships of polyene antibiotics of the amphotericin B group. Antimicrob Agents Chemother. 2013;57(8):3815–22.
Cuddihy G, Wasan EK, Di Y, Wasan KM. The development of oral amphotericin B to treat systemic fungal and parasitic infections: has the myth been finally realized? Pharmaceutics. 2019;11(3):99.
Wijnant GJ, Van Bocxlaer K, Yardley V, Harris A, Alavijeh M, Silva-Pedrosa R, et al. Comparative efficacy, toxicity and biodistribution of the liposomal amphotericin B formulations Fungisome® and Am Bisome® in murine cutaneous leishmaniasis. Int J Parasitol Drugs Drug Resist. 2018;8(2):223–8.
Jain K, Verma AK, Mishra PR, Jain NK. Characterization and evaluation of amphotericin B loaded MDP conjugated poly(propylene imine) dendrimers. Nanomed Nanotechnol Biol Med. 2015;11(3):705–13.
Kajtár M, Vikmon M, Morlin E, Szejtli J. Aggregation of amphotericin B in the presence of γ-cyclodextin. Biopolymers. 1989;28(9):1585–96.
Yu Y, Chen P, Gao M, Lan W, Sun S, Ma Z, et al. Amphotericin B tamed by salicylic acid. ACS Omega. 2022;7(17):14690–6.
Barwicz J, Christian S, Gruda I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob Agents Chemother. 1992;36(10):2310–5.
Parvez S, Yadagiri G, Gedda MR, Singh A, Singh OP, Verma A, et al. Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: an effective oral combination against experimental murine visceral leishmaniasis. Sci Rep. 2020;10(1):12243.
Elzoghby A, Freag M, Mamdouh H, Elkhodairy K. Zein-based nanocarriers as potential natural alternatives for drug and gene delivery: focus on cancer therapy. Curr Pharm Des. 2018;23(35):5261–71.
Hong DY, Lee JS, Lee HG. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int J Biol Macromol. 2016;85:9–15.
Chen S, Li Q, Li H, Yang L, Yi JZ, Xie M, et al. Long-circulating zein-polysulfobetaine conjugate-based nanocarriers for enhancing the stability and pharmacokinetics of curcumin. Mater Sci Eng C. 2020;109: 110636.
Suthar T, Patel P, Singh P, Datusalia AK, Yadav AK, Jain K. Hesperidin microemulsion: formulation optimization, characterization, and in vitro evaluation. J Drug Deliv Sci Technol. 2023;80: 104166.
Maw PD, Pienpinijtham P, Pruksakorn P, Jansook P. Cyclodextrin-based Pickering nanoemulsions containing amphotericin B: Part II Formulation, antifungal activity, and chemical stability. J Drug Deliv Sci Technol. 2022;69:103174.
Soni N, Jain K, Gupta U, Jain NK. Controlled delivery of Gemcitabine Hydrochloride using mannosylated poly(propyleneimine) dendrimers. J Nanopart Res. 2015;17:458. https://doi.org/10.1007/s11051-015-3265-1.
Guhagarkar SA, Malshe VC, Devarajan PV. Nanoparticles of polyethylene sebacate: a new biodegradable polymer. AAPS PharmSciTech. 2009;10:935–42.
Pardhi VP, Suthar T, Sharma A, Jain K. Bedaquiline fumarate microemulsion: formulation optimization, rheological characterization and in vitro studies. Nanomedicine (Lond). 2022;17(21):1529–46. https://doi.org/10.2217/nnm-2022-0132.
Menezes J, Athmaselvi KA. Report on edible films and coatings. In: Food Packaging and Preservation. Cambridge: Academic Press; 2018. p. 177–212.
Wang H, Zhu W, Huang Y, Li Z, Jiang Y, Xie Q. Facile encapsulation of hydroxycamptothecin nanocrystals into zein-based nanocomplexes for active targeting in drug delivery and cell imaging. Acta Biomater. 2017;61:88–100.
Kumar S, Saini V, Maurya IK, Sindhu J, Kumari M, Kataria R, et al. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: potential standalone or adjuvant antimicrobial agents. PLoS One. 2018;13(4): e0196016.
Soto R, Patel P, Albadarin AB, Diniz MO, Hudson SP. Solubility, aggregation and stability of Amphotericin B drug in pure organic solvents: thermodynamic analysis and solid form characterization. J Mol Liq. 2022;366: 120276.
Ng WS, Lee CS, Cheng SF, Chuah CH, Wong SF. Biocompatible polyurethane scaffolds prepared from glycerol monostearate-derived polyester polyol. J Polym Environ. 2018;26:2881–900.
Truzzi E, Capocefalo A, Meneghetti F, Maretti E, Mori M, Iannuccelli V, et al. Design and physicochemical characterization of novel hybrid SLN-liposome nanocarriers for the smart co-delivery of two antitubercular drugs. J Drug Deliv Sci Technol. 2022;70: 103206.
Jansook P, Fülöp Z, Ritthidej GC. Amphotericin B loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): physicochemical and solid-solution state characterizations. Drug Dev Ind Pharm. 2019;45(4):560–7.
AL-Quadeib BT, Radwan MA, Siller L, Horrocks B, Wright MC. Stealth Amphotericin B nanoparticles for oral drug delivery: in vitro optimization. Saudi Pharm J. 2015;23(3):290-302.
Ghanbarzadeh B, Oromiehi AR. Studies on glass transition temperature of mono and bilayer protein films plasticized by glycerol and olive oil. J Appl Polym Sci. 2008;109(5):2848–54.
Lawton JW. Plasticizers for zein: their effect on tensile properties and water absorption of zein films. Cereal Chem. 2004;81(1):1–5.
Shinde P, Agraval H, Srivastav AK, Yadav UCS, Kumar U. Physico-chemical characterization of carvacrol loaded zein nanoparticles for enhanced anticancer activity and investigation of molecular interactions between them by molecular docking. Int J Pharm. 2020;588: 119795.
Singh A, Yadagiri G, Parvez S, Singh OP, Verma A, Sundar S, et al. Formulation, characterization and in vitro anti-leishmanial evaluation of amphotericin B loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate. Mater Sci Eng C. 2020;117: 111279.
Liu Y, Mei Z, Mei L, Tang J, Yuan W, Srinivasan S, et al. Analytical method development and comparability study for Am Bisome® and generic Amphotericin B liposomal products. Eur J Pharm Biopharm. 2020;157:241–9.
Fernández-García R, Muñoz-García JC, Wallace M, Fabian L, González-Burgos E, Gómez-Serranillos MP, et al. Self-assembling, supramolecular chemistry and pharmacology of amphotericin B: poly-aggregates, oligomers and monomers. J Control Release. 2022;341:716–32.
Wang Y, Ke X, Voo ZX, Yap SSL, Yang C, Gao S, et al. Biodegradable functional polycarbonate micelles for controlled release of amphotericin B. Acta Biomater. 2016;46:211–20.
Acknowledgements
The authors thank the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India for providing facilities to write this research article. A.P. is thankful to Indian council of Medical Research (ICMR), New Delhi for the financial support in the form of JRF under the ICMR Extramural Research Project (Project ID: 2020-4686; Ref. No. 5/13/34/2020/NCD-III).
Funding
The National Institute of Pharmaceutical Education and Research-Raebareli (NIPER-R), Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Government of India, provided financial assistance (683/MS-PE/2020).
Author information
Authors and Affiliations
Contributions
K.J. contributed to the conceptualization and supervision and helped to manage resources. Data curation, investigation, and formal analysis were performed by J.N. A.P. contributed to validation, and review and editing.
Corresponding author
Ethics declarations
Competing Interest
The authors clarify that the formulation part of this research work has been filed for Indian patent at Indian Patent Office (IPO), Mumbai, India; Application No.: 202311071032; Date of Application: 18/10/2023.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joyson, N., Pathak, A. & Jain, K. One Platform Comparison of Polymeric and Lipidic Nanoparticles for the Delivery of Amphotericin B. AAPS PharmSciTech 24, 226 (2023). https://doi.org/10.1208/s12249-023-02672-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02672-y