Abstract
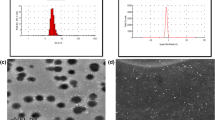
Amphotericin B, a gold standard broad spectrum antibiotic used in treatment of systemic fungal infections and visceral leishmaniasis, though is effective parenterally offers severe nephrotoxicity whereas the oral delivery is reported to give very meager oral bioavailability. Thus, to alleviate the toxicity and to improve oral bioavailability, an effective oral delivery approach in the form of solid lipid nanoparticles of amphotericin B (AmbiOnp) was reported earlier by our group. In this investigation, we report the predominant formation of nontoxic superaggregated form of amphotericin B, resulting from the probe sonication-assisted nanoprecipitation technique. The developed formulation was further confirmed to retain this nontoxic form and was found to be stable over the varied gastrointestinal conditions. Further, in vitro antifungal activity of AmbiOnp against Candida albicans showed minimum inhibitory concentration value of 7.812 μg/mL attributed to controlled release of drug from nanoparticulate matrix. In vivo pharmacokinetic studies revealed a relative bioavailability of AmbiOnp to be 1.05-fold with a Cmax of 1109.31 ± 104.79 ng/mL at the end of 24 h which was comparable to Cmax of 1417.49 ± 85.52 ng/mL achieved with that of marketed formulation (Fungizone®) given intravenously establishing efficacy of AmbiOnp. In vivo biodistribution studies indicated very low levels of Amphotericin B in kidneys when given as AmbiOnp as compared to that of marketed formulation proving its safety and was further corroborated by renal toxicity studies. Further, the formulations were found to be stable under refrigeration condition over a period of 3 months.







Similar content being viewed by others
References
Patel PA, Patravale VB. AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration. J Biomed Nanotechnol. 2011;7(5):632–9.
Wasan KM, Wasan EK, Gershkovich P, Zhu X, Tidwell RR, Werbovetz KA, et al. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J Infect Dis. 2009;200(3):357–60.
Barwicz J, Tancrède P. The effect of aggregation state of amphotericin-B on its interactions with cholesterol- or ergosterol-containing phosphatidylcholine monolayers. Chem Phys Lipids. 1997;85(2):145–55.
Bolard J, Legrand P, Heitz F, Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991;30(23):5707–15.
Italia JL, Yahya MM, Singh D, Ravi Kumar MN. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone. Pharm Res. 2009;26(6):1324–31.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Louria DB. Some aspects of the absorption, distribution, and excretion of amphotericin B in man. Antibiotic Med Clin Ther. 1958;5(5):295–301.
Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. Amphotericin B formulations and drug targeting. J Pharm Sci. 2008;97(7):2405–25.
Gruszecki WI, Gagoś M, Hereć M. Dimers of polyene antibiotic amphotericin B detected by means of fluorescence spectroscopy: molecular organization in solution and in lipid membranes. J Photochem Photobiol B. 2003;69(1):49–57.
Tancrède P, Barwicz J, Jutras S, Gruda I. The effect of surfactants on the aggregation state of amphotericin B. Biochim Biophys Acta. 1990;1030(2):289–95.
Espada R, Valdespina S, Alfonso C, Rivas G, Ballesteros MP, Torrado JJ. Effect of aggregation state on the toxicity of different amphotericin B preparations. Int J Pharm. 2008;361(1–2):64–9.
Duarte M, Giordani RB, De Carli GA, Zuanazzi JA, Macedo AJ, Tasca T. A quantitative resazurin assay to determinate the viability of Trichomonas vaginalis and the cytotoxicity of organic solvents and surfactant agents. Exp Parasitol. 2009;123(2):195–8.
Acknowledgments
The authors are grateful to the Life Care Innovation Pvt. Ltd. (Delhi, India), I.S.P. Technology Pvt. Ltd. (India), Degussa Pvt. Ltd. (France), and BASF (Mumbai, India) for the generous gift samples of drug and excipients. The authors are thankful to the University Grants Commission and Department of Science and Technology, Government of India, New Delhi, India for the financial support extended for the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no competing interests.
Animal studies declaration
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Chaudhari, M.B., Desai, P.P., Patel, P.A. et al. Solid lipid nanoparticles of amphotericin B (AmbiOnp): in vitro and in vivo assessment towards safe and effective oral treatment module. Drug Deliv. and Transl. Res. 6, 354–364 (2016). https://doi.org/10.1007/s13346-015-0267-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0267-6