Abstract
The current study is regarding the development and characterization of Darifenacin-loaded self-assembled liquid crystal cubic nanoparticles (LCCN). An anhydrous approach was used for the preparation of these cubic nanoparticles using a hydrotropic agent (propylene glycol), with minimal energy input. Upon dispersion in aqueous medium, the system was successfully transformed to cubosomal nanoparticles counterpart as depicted by transmission electron micrographs. A Box-Behnken design was used to optimize formulation variables, namely A: amount of GMO, B: amount of Pluronic F127, C: amount of PG, and D: amount of HPMC. The design has generated 29 formulae which were tested regarding drug content uniformity, dispersibility in water, particle size, zeta potential, polydispersity index, and in vitro release behavior. The numerical optimization algorithms have generated an optimized formula with high desirability ≈ 1. The optimized formula displayed small particle size, good homogeneity, and zeta potential along with controlled in vitro release profile and ex vivo permeation through rabbit intestine. Thus, self-assembled LCCN might offer an alternative anhydrous approach for the preparation of cubosomal nanoparticles with controlled release profile for a possibly better control of overactive bladder syndrome which tremendously affect the overall life quality.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An overactive bladder (OAB), according to the International Continence Society (ICS), is defined as urinary urgency, that is may or may not accompanied with incontinence urges, alongside nocturia and frequent urination, usually with the absence of an underlying metabolic or pathologic causes [1, 2]. OAB is distinguished by involuntary urodynamic contractions of bladder’s smooth muscles. Consequently, the patient is unable to subdue such contractions which are prevalent during bladder filling [3]. OAB is a major cause of patient discomfort, with undisputed need for chronic symptomatic treatment. Although OAB has a non-specific age targeting segment, it tends to prevail with elder subjects. Roughly, at least 17 million Americans are likely affected by OAB [4].
It was commonly perceived that women are more likely affected by bladder control problems and its related urinary urgency. This concept was opposed by the National Overactive Bladder Evaluation (NOBLE) Program, which has done an extensive study including over 5000 participants as a representative sample of the US population and the study concluded that the overall prevalence of such condition is likely similar regarding different sex while the severity of symptoms may differ. Nonetheless, OAB disregarding sex difference was found to have a tremendous impact on mental health, sleep quality, and overall life quality. According to the NOBLE program, OAB, with or without urge incontinence, was associated with clinically significant higher Center for Epidemiological Studies Depression Scale “CES-D” scores, poor sleep quality, and overall lower life quality Short Form Health Survey “SF-36” scores among the participants [3].
The muscarinic receptors in human bladder are M2 (80%) and M3 (20%). The M3 receptor, despite being less prevalent, has been denoted as the main receptor, following cholinergic activation, responsible for the contraction of bladder’s smooth muscles [5, 6]. Therefore, antimuscarinic agents are considered the drug of choice for OAB symptomatic treatment. Their mechanism of action is simply blockade of muscarinic receptors resulting in significantly reduced involuntary bladder contractions, leading to better bladder filling and reduced urinary incontinence [7]. Darifenacin hydrobromide (DRF) is a selective potent M3 antagonist, a potentially effective antimuscarinic with enhanced tolerability and reduced safety problems caused by blockade of other non-related muscarinic receptor subtypes [8]. Darifenacin, being very lipophilic, had slow dissolution rate and consequently poor oral bioavailability [9]. Darifenacin was formulated as liposome [10], nanostructured lipid carrier [11], self-emulsifying drug delivery system [12], and β-cyclodextrin inclusion complex buccal films [13] in order to enhance its bioavailability. Best to our knowledge it was not prepared as self-assembled liquid crystal cubic nanoparticles.
Self-assembled liquid crystal cubic nanoparticles (LCCN) introduce a shifted paradigm regarding the newly emerging bi-continuous liquid crystalline nanoparticles known as “cubosomes” which tend to embrace an infinite periodic minimal surfaces contortion distinguished with its zero curvature [14, 15]. Cubic nanoparticles are commonly prepared using glyceryl monooleate (GMO) and Pluronic F127 using homogenizers or ultrasonication to provide high energy input [16,17,18]. Conventional techniques are difficult to scale up and posing risk of disrupting the cubic arrangement due to excessive energy input [19]. LCCN are considered the anhydrous precursor formulated using a hydrotrope which upon mixing with aqueous medium transform to their counterpart cubosomes with minimal energy input, thus presenting a more stable and versatile alternative technique of preparation [20]. Drugs loaded in cubosomes displayed a sustained release pattern due to the composite diffusion pathway caused by the offered large interfacial area. Also, the lipid backbone monoolein is biocompatible, bioadhesive, and digestible as it is subjected to lipolysis [21, 22].
Self-assembled LCCN provide an excellent oral delivery carrier for hydrophobic drugs. As a lyotropic system, they can accommodate hydrophobic drugs in a solubilized state within their lipid bilayers [23]. Additionally, they can retain such solubilized state without drug precipitation even after their digestion by intestinal lipases since the cubic phase is isotropic. Also, they enhance drug absorption by prolonging the drug contact time with intestinal membrane as they are bioadhesive. Moreover, cubic nanoparticles provided enhanced transdermal and transmucosal delivery which could be expected to be mimicked in oral delivery as well [24].
Based upon the abovementioned data, the self-assembled liquid crystal cubic nanoparticles were chosen to enhance DRF bioavailability as well as to confer a controlled release pattern by adopting a new anhydrous approach with the aim of enhancing the system’s physical stability through using minimal energy input during the preparation to avoid the disruption of cubosomal arrangement. A Box-Behnken design was adopted in the preparation of DRF-LCCN to scrutinize the effect of formulation variables on drug content uniformity, dispersibility in water, particle size, zeta potential, polydispersity index, and in vitro release behavior. Moreover, numerical optimization algorithms were used to suggest an optimized formula, which was consequently prepared and inspected using transmission electron microscopy to visualize the system morphology after reconstitution with water and finally, tested for its ex vivo permeation performance through rabbit intestine as a measure for bioavailability enhancement.
Materials and Methods
Materials
Darifenacin hydrobromide (DRF) was received as a kind gift from Marcyrl Pharmaceutical Industries, Egypt. Hydroxypropyl Methylcellulose E5 (HPMC E5, average molecular weight is 10,000 Daltons) was purchased from Dow Chemical Company Midland, USA. Propylene glycol (PG) and absolute ethanol were obtained from Elnasr Chemicals, Egypt. Glyceryl monooleate (monoolein, GMO) was a gift from Danisco Cultor, Grindsted, Denmark. Pluronic F127 was procured from Sigma-Aldrich Co., St. Louis, USA. Spectra/Por® dialysis membrane (12,000–14,000 Daltons cut-off) was brought from Spectrum Laboratories Inc. (USA). All other reagents and chemicals used were of analytical reagent grade.
Preparation of DRF-LCCN
For the preparation of DRF-LCCN, Chung et al. [20] technique was adopted with some modifications; accurate weights of GMO and Pluronic F127 were mixed in a beaker containing 20 ml of absolute ethanol and placed in an oven set at 50°C till complete dissolution. Then, an accurate weight of DRF (100 mg) was added to the solution and returned to the oven till it is completely dissolved. After that, an accurate weight of HPMC was sprinkled and mixed with the lipid solution.
Consequently, the mixture was evaporated under vacuum (Hei-VAP Expert Heidolph Instruments GmbH & Co., Schwabach, Germany) till complete evaporation of ethanol. A viscous liquid was formed, which was diluted with a specified volume of absolute ethanol either with or without PG to be mixed together in the rotavap for 15 min to form a homogenous DRF-LCCN liquid dispersion.
Experimental Design
Design Expert® software (Version 10, Stat-Ease Inc., MN, USA) was used for the statistical optimization of formulation variables, implementing a Box-Behnken design generating 29 runs listed in standard order (Table I). Four factors were studied as independent variables, namely, A: amount of GMO, B: amount of Pluronic F127, C: amount of PG, and D: amount of HPMC (Table II). The particle size (Y1), quantity released at 1 h (Y2), quantity released at 4 h (Y3), quantity released at 8 h (Y4), and quantity released at 24 h (Y5) were the target responses (Table II). The desirability function was applied to seek an optimal formula by applying constraints for the optimization of determinants using the fitted polynomial equations generated by the software.
DRF-LCCN Characterization
Drug Content Uniformity
DRF was measured using a modified RP-HPLC reported technique [25]. The method was validated regarding its accuracy, precision, selectivity, and linearity. The HPLC system was composed of a ZORBAX SB-C18 column 150 × 4.6 mm, particle size: 5 μm kept at ambient temperature (Agilent technologies, Santa Clara, CA), LaChrom D7000 integrator, L-7110 pump, and UV-VIS L-7420 detector (Hitachi, Tokyo, Japan). The mobile phase consisted of acetonitrile: acetate buffer (100 g of ammonium acetate + 4.1 ml of glacial acetic acid in 0.5 l deionized water) containing 2 ml triethylamine (50:50% v/v) and the pH was adjusted to 6 using acetic acid. The analysis was done using an isocratic flow rate of 1 ml/min; chromatograms were recorded at λmax = 230 nm.
An accurate weight of each formula equivalent to 10 mg DRF was dissolved in 10 ml methanol for 10 min. Then, the solution was filtered through 0.22-µm nylon filter membrane and subsequently injected into the abovementioned HPLC system for quantification.
Dispersibility Test
The dispersibility test was carried out to determine the ability of self-assembled LCCN to form cubosomal homogenous dispersion upon dispersion in water. The test was performed by placing a beaker containing 20 ml distilled water on a magnetic stirrer at 37 ± 0.5°C to which a known volume (0.1 ml) of each formula was added and stirred at 500 rpm. The ease of dispersion criterion was visually assessed via observing the dispersion medium and calculating the time for complete dispersibility for all formulae. The formula was considered easily dispersed or able to form self-assembled cubosomal homogenous dispersion if completely dispersed in less than 30 min forming clear dispersion without oily globules on surface and no particles were deposited at the bottom [26].
Particle Size, Zeta Potential, and Polydispersity Index (PDI) Determination
An amount of DRF-LCCN equivalent to 5 mg drug was dispersed in 10 ml of distilled water, vortexed for 5 min then diluted in a ratio of 1:10, and subjected to light scattering at 173° in order to measure the vesicles size (z-average) and the polydispersity index (PDI) which are based on the particles’ Brownian motion that caused fluctuation of the scattered light. The zeta potential (ζ) was also measured adopting electrophoresis principle. All results were measured using Zetasizer Nano ZS (model MAM 5000, Malvern instrument limited, Worcestershire, UK) [27].
In Vitro Release Study of DRF-LCCN
In order to study the drug release from the prepared LCCN, a dialysis method was applied using Spectra/Por® dialysis membrane (12,000–14,000 molecular weight cut-off). The membrane was soaked for 1 h in distilled water to remove any preservative traces before use [28]. The membrane was tied with a thread from both ends like a sac to be filled with DRF-LCCN dispersion. A known volume (equivalent to 5 mg DRF) was dispersed in 2 ml of distilled water, vortexed, and then filled in the dialysis membrane sac. The release was done in 100 ml phosphate buffer saline (PBS, pH = 7.4), in a glass bottle placed in a thermostatically controlled water bath (Model 1083; GLF Corp., Burgwedel, Germany) set at 37°C and 50 strokes per min. At predetermined time intervals (1, 2, 4, 6, 8, and 24 h), 3 ml of the release medium was withdrawn for analysis and replenished with equal volume of release medium to maintain constant volume. Then, aliquots were filtered through 0.22-µm nylon filter membrane and subsequently injected into the abovementioned HPLC system for quantification. Results are the mean values of three in vitro release experiments [18].
Transmission Electron Microscopy (TEM)
The optimized DRF-LCCN formula equivalent to 5 mg drug was dispersed in 5 ml distilled water to envision the morphological shape of the cubosomal vesicles using TEM (Joel JEM 1400, Tokyo, Japan). The dispersion was furtherly diluted in a ratio of 1:10 with distilled water and filtered through Whatman qualitative filter paper (pore size 11 µm) to remove any aggregates. Consequently, known amount of 1% phosphotungstic acid was added and mixed. Then, the carbon-coated copper grid was impregnated with one drop of the dispersion, allowing drainage of excess sample and the grid was left to dry at ambient temperature till examination [29, 30].
Ex Vivo Permeation Study of DRF-LCCN
The ex vivo permeation study, approved by Research Ethics Committee (REC), Faculty of Pharmacy, Cairo University (approval number PI 1166), was done to test the ability of the prepared DRF-LCCN to penetrate through the intestinal barrier. Adult male Albino rabbit’s intestine was used for this test. The excised rabbit intestine was thoroughly washed with saline before use, cleaned from intestinal residues, and divided into segments (8 cm long). The intestinal segments were tied at both ends and known volumes of the optimized DRF-LCCN or drug suspension equivalent to 5 mg DRF were filled inside different segments. Then, the segments were immersed in closed glass bottles containing 100 ml PBS (pH = 7.4). The glass bottles were placed in a thermostatically controlled (37 ± 0.5°C) shaking water bath (Model 1083; GLF Corp., Burgwedel, Germany). Aliquots (1 ml) were withdrawn at predetermined time intervals (0.5, 1, 2, 4, 6, 8, and 24 h) and were replenished with fresh medium. The aliquots were filtered through 0.22-µm nylon filter membrane and subsequently injected into the abovementioned HPLC system for quantification. The ex vivo permeation experiment was done in triplicates [31].
Results and Discussion
DRF-LCCN Characterization
Drug Content Uniformity
Regarding the uniformity of drug content of the prepared self-assembled DRF-LCCN, it was ranged from 90.11 ± 1.18 to 109.87 ± 2.07% as shown in Table I indicating the uniformity of preparation technique.
Dispersibility Test
The dispersibility test was conducted to test the ease of dispersion of the prepared self-assembled DRF-LCCN upon dispersion in water to form a homogenous isotropic mixture. As shown in Table I, the time for dispersion of the prepared self-assembled DRF-LCCN ranged from 4 ± 0.5 to 68 ± 12 s which indicated the spontaneity and ease of dispersion of the prepared formulae as they required very short time (less than 2 min) for complete dispersion. All formulae produced transparent dispersion and few of them were slightly gelatinous in nature. It was noticed that formulae containing high GMO (LCCN 15 and 16) exhibited long dispersibility time (65 and 68 s respectively) which could be due to lipophilic nature of GMO which hindered dispersibility. Meanwhile, formulae containing high HPMC (LCCN 11) exhibited moderate dispersibility time around 25 s which could be attributed to the imparted viscosity which slightly prolonged dispersibility time. On the other hand, formulae containing high GMO and HPMC with high pluronic and PG displayed short dispersibility time (LCCN 6) 7 s. This is probably due to the good emulsifying and stabilizing property of pluronic which is a water soluble amphiphilic surfactant with high HLB along with the cosolvent role of propylene glycol both exerted a synergistic effect on emulsifying, stabilizing, and solubilizing GMO when dispersed in water [30, 32].
Particle Size, Zeta Potential, and Polydispersity Index Determination
Particle size is a crucial parameter directly affecting drug absorption and bioavailability; thus, nanosized particles will greatly enhance the oral bioavailability of poorly absorbed drug [33]. The average particle size of the dispersed self-assembled LCCN ranged from 208.68 ± 15.55 to 810.62 ± 54.87 nm as shown in Table I which confirmed the nanosize of all prepared systems upon dispersion in distilled water as shown in Table I. Statistical analysis revealed that the quadratic model is fitted; both amount of Pluronic F127 (B) and amount of HPMC (D) have significant effect (P = 0.0022 and 0.0027 respectively) on particle size. Pluronic F127 had negative effect on particle size as shown in Fig. 1a which means that increasing the added amount of pluronic from 5 to 27.5 mg will reduce the particle size while further increase from 27.5 to 50 mg will slightly increase the particle size. These findings are compatible with results obtained by Nadia et al. [18], Esposito et al. [34], and Adam et al. [35]. This is probably due to the ability of hydrophilic copolymer to break large aggregates into smaller particles owing to their surface activity and stabilizing effect of polyethylene oxide (PEO) moiety while increasing their concentration may exceed their critical micelle concentration (CMC) resulting in micellar configuration which has no surface activity resulting in larger particle size [36]. Regarding HPMC, it has a positive effect on the particle size as shown in Fig. 1a; this means that increasing HPMC amount increases the particle size possibly due to its ability to impart viscosity, which is directly proportional to its molecular weight, resulting in the formation of larger particles [37]. Also, HPMC could form intermolecular complexes with pluronic through formation of inter molecular hydrogen bond and hydrophobic associations between the polyoxyethylene moiety of pluronic and cellulose backbone of HPMC resulting in larger particle size [38].
a 3D plot showing the effect of Pluronic F127 and HPMC on the particle size of the self-assembled liquid crystal cubic nanoparticles. b Interaction plot showing the antagonistic interaction between GMO and PG on the percentage released after 1 h. c Interaction plot showing the antagonistic interaction between Pluronic F127 and HPMC on the percentage released after 1 h. d One factor plot showing the negative effect of HPMC on the percentage released after 4 h
The prepared self-assembled LCCN exhibited satisfactory zeta potential ranging from − 6.68 ± 0.42 to − 21.25 ± 3.16 mV as shown in Table I. The negative charge is probably due to the carboxylic groups of free fatty acids associated with GMO [39, 40]. Moreover, most formulae demonstrated good homogeneity (PDI value less than 0.5) reflected through their low PDI ranging from 0.12 ± 0.04 to 0.32 ± 0.07 as presented in Table I indicating the homogeneity of the prepared systems [41, 42].
In Vitro Release Study of DRF-LCCN
The in vitro release profiles of the prepared self-assembled LCCN are illustrated in Fig. 2. The release profiles were statistically analyzed at 4 different time points Q1, Q4, Q8, and Q24 respectively in order to determine and optimize the formulation variables to achieve a controlled release profile. ANOVA study revealed that linear model was fitted in Q4 and Q24 while two factor interactions model was fitted in Q1 and quadratic model was fitted in Q8. Statistical analysis indicated that PG had a significant positive effect on the release at the 4 different time points (P = 0.0139, 0.0039, 0.0002, and 0.007 respectively) as shown in Figs. 1b and 3a–d respectively, which is possibly due to the fact that it acts as a cosolvent for GMO thus enhancing its solubility with aqueous medium, and may be also attributed to its ability to reduce the formulation viscosity thus allowing more entrapped drug to be released from the cubic phase. Similar results were found by Lim DG et al. for the release of sodium fluorescein [43].
a Interaction plot showing the antagonistic interaction between GMO and PG on the percentage released after 4 h. b 3D plot showing the effect of Pluronic F127 and HPMC on the percentage released after 8 h. c 3D plot showing the effect of Pluronic F127 and PG on the percentage released after 8 h. d One factor plot showing the effect of PG on the percentage released after 24 h
Regarding HPMC, it was found to have a negative effect on the release at Q1, Q4, and Q8 as shown in Figs. 1c, d and 3b respectively, possibly due to the imparted barrier caused by HPMC, a polymer known for its high viscosity; as it increases the viscosity of the system resulting in reduced drug release, the glassy barrier caused by HPMC affected the release till Q8; then, it seems that after the complete hydration of this glassy barrier, the release was not controlled by HPMC anymore; thus, it did not have a significant effect on the release at Q24 [44, 45]. Similar findings were also observed by Gehrke and Lee as they found that the barrier effect of HPMC last for 4–6 h depending on drug solubility [46].
Also, an antagonistic interaction was found between GMO and PG on Q1 and Q4 as illustrated in Figs. 1b and 3a respectively; GMO represents the hydrophobic backbone of the cubic phase; thus in absence of PG, further increase of GMO will reduce the release of DRF which is entrapped inside the cubic phase structure. But in presence of PG cosolvent, GMO possibly forms a bi-continuous lipid water system called liquid sponge phase which has better diffusion compared to cubic phase due to formation of hydrophilic channel [47, 48].
ANOVA study has also revealed another antagonistic interaction between HPMC and Pluronic F127 on Q1 and Q8 as illustrated in Figs. 1c and 3b respectively, which means that at high HPMC level, increasing pluronic concentration resulted in reduced drug release which could be explained through the fact that at low pluronic concentration, most of the surfactant is present at the surface of cubic structure while at high concentration, most of the surfactant will integrate in the cubic bulk structure thus reducing drug release and stabilize the drug inside the cubic phase by the help of the high viscosity imparted by HPMC while at low HPMC level, the opposite is observed as the medium become less viscous and the cubic phase is not well maintained thus increasing pluronic concentration will act as solubilizer rather than incorporation inside the cubic phase matrix thus more drug will be released [49].
Another finding revealed by ANOVA study is that Pluronic F127 had a significant positive effect on the release at Q8; this could be possibly related to the enhanced solubilization into the aqueous release medium owing to the good solubilizing effect of pluronic and to the reduction of particle size which allowed more surface area for drug release [50, 51]. Moreover, there is a synergistic interaction between PG and Pluronic F127 as illustrated in Fig. 3c, which is kind of expected as both have positive effect on the release at Q8 which was discussed earlier.
Statistical Optimization of the Prepared DRF-LCCN
The Design Expert software was used to optimize the formulation variables by applying the constraints mentioned in Table II in order to obtain a controlled release profile with particle size less than 500 nm which was found ideal for intestinal absorption [52, 53]. The numerical optimization function, through the generated fitted polynomial equation as shown in Table III after omitting non-significant model terms, has generated an optimum formulation with desirability = 1. The composition of the optimal formula was 441.69 mg GMO, 45.88 mg Pluronic F127, 0.12 ml PG, 93.7 mg HPMC, and 10 mg DRF. The optimized formula was prepared and characterized as shown in Table I where it showed small particle size (less than 500 nm) = 324.08 ± 24.68 nm (Y1). The in vitro release profile of the optimized formula showed a controlled release of DRF where Q1 = 20.78% ± 2.40% (Y2), Q4 = 44.31% ± 5.03% (Y3), Q8 = 50.42% ± 0.92% (Y4), and Q24 = 74.90% ± 0.84% (Y5) as shown in Fig. 2d.
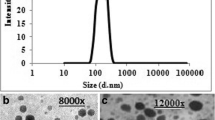
Transmission Electron Microscopy
The transmission electron microscopy test was performed to confirm the data previously obtained from zeta sizer and to visualize the ability of the optimized self-assembled LCCN to produce cubosomal vesicles upon dispersion in water [19]. As shown in Fig. 4, the captured micrographs demonstrated the nearly perfect cubic multi-angular shape of the vesicles which are well differentiated and with no visible aggregates. The measured size of random vesicles is consistent with zeta sizer measurements.
Ex Vivo Permeation Study of DRF-LCCN
The permeation profiles of DRF from the optimized self-assembled LCCN and drug suspension are illustrated in Fig. 5. The optimized self-assembled LCCN showed lower cumulative amount permeated of DRF after 8 h (Q8hr = 775 μg/cm2) relative to that permeated from drug suspension (Q8hr = 3336 μg/cm2) while after 24 h, the cumulative amount permeated from optimized formula (Q24hr = 2978 μg/cm2) was still less than that permeated from drug suspension (Q24hr = 3799 μg/cm2) indicating the ability of the optimized formula to provide better sustainment of DRF throughout the entire day compared to drug suspension.
The reduced cumulative amount permeated owed to the better sustainment of the optimized self-assembled LCCN does not compromise the bioavailability of DRF. The cubic nanoparticles have the merits of being digestible by intestinal lipases forming drug-loaded lyotropic nanostructures [54]. These nanostructures are capable of penetrating the aqueous boundary layer adjacent to the intestinal membrane, keeping the drug in closer contact with the endothelial membrane for better internalization through endocytosis, thus providing enhanced absorption and better uptake [55].
These findings might indicate the potentiality of self-assembled LCCN as a novel system to control drug release over a long period of time. Such release pattern could allow prolonged contact time with intestine resulting in better permeation and enhanced oral bioavailability. Also being a lyotropic system, DRF was kept in a solubilized state without the formation of any precipitates in the permeation aqueous medium despite DRF lipophilicity. The capacity of this novel system to keep lipophilic drug in a solubilized state while controlling their release alongside the ease of preparation and its better stability is the ultimate combination for an ideal sustained release system.
Conclusion
Self-assembled LCCN is a novel anhydrous approach for the preparation of cubic nanoparticles which was successfully prepared and tested for its dispersibility in aqueous medium to transform into cubosomal nanoparticles with minimal energy input. Cubosomes have promising future prospects as drug delivery systems due to their ability to encapsulate hydrophilic and hydrophobic drugs, high biocompatibility and stability. They can also be functionalized with targeting ligands and used in other industries like cosmetics, sunscreens, and food. However, further research is needed to up-scale their use in different applications. The prepared self-assembled LCCN showed nano-scaled particles size, acceptable zeta potential, and good homogeneity along with controlled release profile for DRF. The adopted Box-Behnken design generated an optimized formula which displayed a perfect zero curvature structure under TEM and controlled permeation through rabbit intestine. Thus, we presume that the anhydrous technique of preparation of self-assembled LCCN using hydrotropic agent could present a revolutionary yet simple approach for the preparation of stable cubosomal nanoparticles. The self-assembled LCCN provided controlled in vitro release and ex vivo permeation profiles for DRF which might possibly enhance the overnight control of overactive bladder syndrome for a better life quality.
Data Availability
Raw data will be made available upon request.
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187(1):116–26.
Milsom I, Stewart W, Thuroff J. The prevalence of overactive bladder. Am J Manag Care. 2000;6(11 Suppl):S565–73.
Stewart W, Van Rooyen J, Cundiff G, Abrams P, Herzog A, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–36.
Abrams P, Kelleher C, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6(11 Suppl):S580–90.
Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45(4):420–9.
Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002;22(3):133–45.
Andersson K-E. Potential benefits of muscarinic M3 receptor selectivity. Eur Urol Suppl. 2002;1(4):23–8.
Wallis RM, Napier CM. Muscarinic antagonists in development for disorders of smooth muscle function. Life Sci. 1999;64(6–7):395–401.
Chapple CR. Darifenacin: a novel M3 muscarinic selective receptor antagonist for the treatment of overactive bladder. Expert Opin Investig Drugs. 2004;13(11):1493–500.
Latha K, Lalitha P, Nasseb BS. Formulation and evaluation of darifenacin hydrobromide nano-liposomes. J Chem Pharm Res. 2017;9(8):174–6.
Hussein AA. Preparation and evaluation of darifenacin hydrobromide loaded nanostructured lipid carriers for oral administration. Iraqi J Pharm Sci. 2018;27(1):53–68.
SreeHarsha N, Shariff A, Shendkar YA, Al-Dhubiab BE, Meravanige G. Development and evaluation of a (SEDDS) self—emulsifying drug delivery system for darifenacin hydrobromide. Indian J Pharm Educ Res. 2019;53:s204–12.
Jagdale SC, Mohanty P, Chabukswar AR, Kuchekar BS. Dissolution rate enhancement, design and development of buccal drug delivery of darifenacin hydroxypropyl β-cyclodextrin inclusion complexes. J Pharm. 2013;2013:983702. https://doi.org/10.1155/2013/983702.
Larsson K. Colloidal dispersions of ordered lipid-water phases. J Dispersion Sci Technol. 1999;20(1–2):27–34.
Bei D, Marszalek J, Youan B-BC. Formulation of dacarbazine-loaded cubosomes—part I: influence of formulation variables. Aaps PharmScitech. 2009;10(3):1032–9.
Lai J, Lu Y, Yin Z, Hu F, Wu W. Pharmacokinetics and enhanced oral bioavailability in beagle dogs of cyclosporine A encapsulated in glyceryl monooleate/poloxamer 407 cubic nanoparticles. Int J Nanomed. 2010;5:13.
Um JY, Chung H, Kim KS, Kwon IC, Jeong SY. In vitro cellular interaction and absorption of dispersed cubic particles. Int J Pharm. 2003;253(1–2):71–80.
Morsi NM, Abdelbary GA, Ahmed MA. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: development and in vitro/in vivo characterization. Eur J Pharm Biopharm. 2014;86(2):178–89.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19):5748–56.
Chung H, Kim J-s, Um J, Kwon IC, Jeong S. Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia. 2002;45(3):448–51.
Barauskas J, Johnsson M, Joabsson F, Tiberg F. Cubic phase nanoparticles (cubosome): principles for controlling size, structure, and stability. Langmuir. 2005;21(6):2569–77.
Almgren M, Rangelov S. Polymorph dispersed particles from the bicontinuous cubic phase of glycerol monooleate stabilized by PEG-copolymers with lipid-mimetic hydrophobic anchors. J Dispersion Sci Technol. 2006;27(5):599–609.
Barauskas J, Johnsson M, Tiberg F. Self-assembled lipid superstructures: beyond vesicles and liposomes. Nano Lett. 2005;5(8):1615–9.
Garg G, Saraf S, Saraf S. Cubosomes: an overview. Biol Pharm Bull. 2007;30(2):350–3.
Acharya A, Satish N, Manoj L, Chitti R. Development and validation of RP-HPLC method for the determination of Darifenacin Hydrobromide in bulk drug and pharmaceutical dosage form. Kathmandu Univ J Sci Eng Technol. 2017;13(1):36–44.
Jannin V, Chevrier S, Michenaud M, Dumont C, Belotti S, Chavant Y, et al. Development of self emulsifying lipid formulations of BCS class II drugs with low to medium lipophilicity. Int J Pharm. 2015;495(1):385–92.
Farag MM, Abd El Malak NS, Yehia SA, Ahmed MA. Hyaluronic acid conjugated metformin-phospholipid sonocomplex: a biphasic complexation approach to correct hypoxic tumour microenvironment. Int J Nanomed. 2021;16:1005.
Karakatsani M, Dedhiya M, Plakogiannis FM. The effect of permeation enhancers on the viscosity and the release profile of transdermal hydroxypropyl methylcellulose gel formulations containing diltiazem HCl. Drug Dev Ind Pharm. 2010;36(10):1195–206.
Farag MM, Abd El Malak NS, Yehia SA, Ahmed MA. Sonocomplexation as an effective tool to enhance the antitumorigenic effect of metformin: preparation, in vitro characterization, molecular dynamic simulation & MiaPaCa-2 cell line hypoxia evaluation. J Drug Deliv Sci Technol. 2020;59:101968.
Al-Mahallawi AM, Abdelbary AA, El-Zahaby SA. Norfloxacin loaded nano-cubosomes for enhanced management of otitis externa: In vitro and in vivo evaluation. Int J Pharm. 2021;600.
Sayed S, Habib BA, Elsayed GM. Tri-block co-polymer nanocarriers for enhancement of oral delivery of felodipine: preparation, in vitro characterization and ex vivo permeation. J Liposome Res. 2018;28(3):182–92.
Basha M, Abd El-Alim SH, Shamma RN, Awad GE. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J Liposome Res. 2013;23(3):203–10.
Delie F. Evaluation of nano-and microparticle uptake by the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34(2–3):221–33.
Esposito E, Eblovi N, Rasi S, Drechsler M, Di Gregorio GM, Menegatti E, et al. Lipid-based supramolecular systems for topical application: a preformulatory study. AAPS PharmSci. 2003;5(4):62–76.
Tilley AJ, Drummond CJ, Boyd BJ. Disposition and association of the steric stabilizer Pluronic® F127 in lyotropic liquid crystalline nanostructured particle dispersions. J Colloid Interface Sci. 2013;392:288–96.
Chong JY, Mulet X, Waddington LJ, Boyd BJ, Drummond CJ. Steric stabilisation of self-assembled cubic lyotropic liquid crystalline nanoparticles: high throughput evaluation of triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymers. Soft Matter. 2011;7(10):4768–77.
Battaglia L, Gallarate M, Cavalli R, Trotta M. Solid lipid nanoparticles produced through a coacervation method. J Microencapsul. 2010;27(1):78–85.
da Silva JB, Dos Santos RS, da Silva MB, Braga G, Cook MT, Bruschi ML. Interaction between mucoadhesive cellulose derivatives and Pluronic F127: Investigation on the micelle structure and mucoadhesive performance. Mater Sci Eng C. 2021;119.
Salah S, Mahmoud AA, Kamel AO. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017;24(1):846–56.
Han S, Shen J-q, Gan Y, Geng H-m, Zhang X-x, Zhu C-l, et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin. 2010;31(8):990–8.
Ahirrao M, Shrotriya S. In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. Drug Dev Ind Pharm. 2017;43(10):1686–93.
Vasanth S, Dubey A, GS R, Lewis SA, Ghate VM, El-Zahaby SA, et al. Development and investigation of vitamin C-enriched adapalene-loaded transfersome gel: a collegial approach for the treatment of acne vulgaris. AAPS PharmSciTech. 2020;21(2):1–17.
Lim DG, Jeong W-W, Kim NA, Lim JY, Lee S-H, Shim WS, et al. Effect of the glyceryl monooleate-based lyotropic phases on skin permeation using in vitro diffusion and skin imaging. Asian J Pharm Sci. 2014;9(6):324–9.
Nokhodchi A, Aliakbar R, Desai S, Javadzadeh Y. Liquisolid compacts: the effect of cosolvent and HPMC on theophylline release. Colloids Surf B. 2010;79(1):262–9.
Bettini R, Catellani P, Santi P, Massimo G, Peppas N, Colombo P. Translocation of drug particles in HPMC matrix gel layer: effect of drug solubility and influence on release rate. J Control Release. 2001;70(3):383–91.
Gehrke S, Lee P. Hydrogels for drug delivery systems. Drugs Pharm Sci. 1990;41:333–92.
Alfons K, Engstrom S. Drug compatibility with the sponge phases formed in monoolein, water, and propylene glycol or poly (ethylene glycol). J Pharm Sci. 1998;87(12):1527–30.
Lee J, Kellaway IW. In vitro peptide release from liquid crystalline buccal delivery systems. Int J Pharm. 2000;195(1–2):29–33.
Zhao XY, Zhang J, Zheng LQ, Li DH. Studies of cubosomes as a sustained drug delivery system. J Dispersion Sci Technol. 2005;25(6):795–9.
Nasr M, Dawoud M. Sorbitol based powder precursor of cubosomes as an oral delivery system for improved bioavailability of poorly water soluble drugs. J Drug Deliv Sci Technol. 2016;35:106–13.
Mei L, Xie Y, Jing H, Huang Y, Chen J, Ran H, et al. A novel design for stable self-assembly cubosome precursor-microparticles enhancing dissolution of insoluble drugs. Drug Dev Ind Pharm. 2017;43(8):1239–43.
Florence AT. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 2004;12(2):65–70.
Fonte P, Nogueira T, Gehm C, Ferreira D, Sarmento B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv Transl Res. 2011;1(4):299–308.
Lai J, Chen J, Lu Y, Sun J, Hu F, Yin Z, et al. Glyceryl monooleate/poloxamer 407 cubic nanoparticles as oral drug delivery systems: I. In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. Aaps Pharmscitech. 2009;10(3):960–6.
Yang Z, Peng X, Tan Y, Chen M, Zhu X, Feng M, et al. Optimization of the preparation process for an oral phytantriol-based amphotericin B cubosomes. J Nanomater. 2011;2011:1–10. https://doi.org/10.1155/2011/308016.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Data collection, interpretation, and drafting of the manuscript were carried out by Michael M. Farag and Wessam El-Sebaie, while Emad B. Basalious and Omaima N. El-Gazayerly participated in designing the study, performing statistical analysis of the data, and making significant revisions to the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, M.M., El-Sebaie, W., Basalious, E.B. et al. Darifenacin Self-assembled Liquid Crystal Cubic Nanoparticles: a Sustained Release Approach for an Overnight Control of Overactive Bladder. AAPS PharmSciTech 24, 120 (2023). https://doi.org/10.1208/s12249-023-02575-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02575-y