Abstract
The aim of the current study is the development of a vitamin D3 (VD3)-loaded nanoemulsion (NE) formulation to improve VD3 oral bioavailability for management of vitamin D inadequacy in autistic children. Eight NE formulations were prepared by high-speed homogenization followed by ultrasonication. Four vegetable oils were employed along with two concentrations of Span 20 as the emulsifier. Glycerol, fructose, and mango flavor were included as viscosity modifier, sweetening, and flavoring agents, respectively. The prepared VD3-loaded NE formulations exhibited high drug content (> 98%), droplet size (DS) ranging from 61.15 to 129.8 nm with narrow size distribution, zeta potential values between − 9.83 and − 19.22 mV, and acceptable pH values (4.59–5.89). Storage stability showed that NE formulations underwent coalescence and phase separation during 6 months at room temperature, whereas at refrigerated conditions, formulations showed slight creaming. The optimum formulation (VD3-NE6) revealed a non-significant DS growth at refrigerated conditions and spherical morphology under transmission electron microscopy. VD3-NE6 did not produce any toxic effects to rats treated orally for 3 months, where normal blood picture and kidney and liver functions were observed compared to control rats. Also, serum calcium, oxidative stress, and apoptosis biomarkers remained within normal levels, indicating the safety of the optimum formulation. Furthermore, evaluation of VD3-NE6 oral bioavailability depicted a significant increase in AUC0–72 and Cmax with decreased Tmax compared to plain VD3. The optimum formulation demonstrated improved stability, safety, and oral bioavailability indicating the potential for successful management of vitamin D deficiency in autistic children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies reported that patients with autism spectrum disorder (ASD) suffer from nutritional and metabolic anomalies such as mitochondrial dysfunction, methylation impairment and oxidative stress [1]. The etiology of ASD is still uncertain in the majority of cases; nevertheless, combinations of various environmental and genetic aspects are potentially contributing factors [2]. Evidentially, mineral and vitamin supplementation could assist these vital physiologic processes [1]. As one important factor of the proposed pathogenesis of autism, nutrient insufficiencies are of specific concern. Moreover, the characteristics and behavior of ASD and autistic particular involvements could escalate the hazard of sub-optimal nutrition [3]. Autistic children are generally selective with restricted food preferences based on sensory modalities such as taste, smell, and texture [4]. A recent study highlighted the contribution of palatability to food acceptance among those children [5]. Moreover, the gastrointestinal abnormalities in autism impair the absorption of micronutrients [6]. Consequently, the threat of nutrient insufficiency is becoming of scientific importance [7]. Nutrient supplementation can be highly effective with no adverse effects, as no molecules foreign to the body are used [8].
Vitamin D is a fat-soluble micronutrient which does a vital task in skeletal functions, e.g., bone health and calcium absorption in addition to non-skeletal functions like inhibiting diabetes, cardiovascular diseases, and cancers [9, 10]. The deficiency of vitamin D is considered one of the most widespread micronutrient malnutrition disorders [11, 12]. Its inadequate intake could lead to osteoporosis, rickets, calcium–phosphorus imbalance, parathyroid imbalance, and diabetes [13]. Chemically, vitamin D has two major forms, namely, ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) [14]. In humans, vitamin D3 (VD3) has more potency compared to vitamin D2 owing to its greater efficiency to raise blood serum levels of the bioactive metabolites of vitamin D [15]. Upon exposure to UV radiation, VD3 is naturally synthesized in human skin cells. However, due to low exposure to sunlight and/or poor diet in many countries, vitamin D deficiency is still widespread [9, 16].
Recently, vitamin D has gained attention in research concerning the field of psychiatry [3]. Current studies have revealed a significantly lower vitamin D level in autistic children compared to healthy ones [17,18,19,20]. Accordingly, the supplementation of vitamin D for autistic children is necessary [21]. VD3 is highly sensitive to environmental stresses and is liable to oxidation which leads to losing its physiological benefits and functionality [14]. It also exhibits poor water solubility and oral bioavailability (44.8%) [22]. Being a fat-soluble vitamin, it is commonly absorbed with fatty acids and fats at particular sites in the small intestine via both active and passive transport [23]. Thus, the use of lipid-based drug delivery systems for encapsulation of VD3 would essentially lead to an enhancement of its bioavailability [24, 25]. Moreover, encapsulating VD3 in these delivery systems will also enable its release in a controlled manner and allow the administration of optimal doses, thus avoiding potential side effects of hypervitaminosis syndrome [13].
Nanoparticulate systems loaded with drugs, also called nanodrugs or nanomedicines, have shown remarkable outcomes linked to the absence of collateral toxicities and improved drug efficacy [26]. Nanoemulsion (NE) is a lipid-based drug delivery system that mainly consists of oily phase, aqueous phase, and surfactant [27]. It is a suitable way for nanodispersion of lipophilic bioactives in aqueous environments to be further used in foods and pharmaceuticals [28, 29]. NEs are biocompatible, biodegradable, simple to fabricate, and employed as platforms for hydrophobic therapeutic agents that undergo hydrolysis [30]. Compared to conventional emulsions, NE droplets are characterized by their small size that potentially enhance their stability to coalescence, gravitational separation, and flocculation [31]. NEs reveal outstanding drug release profile because of the big interfacial area [32]. It has been also proposed that the smaller the NE droplet size, the higher the bioavailability of encapsulated lipophilic compounds [33,34,35]. They may also be administered through different routes depending on their intended application [36].
Accordingly, this work aims to formulate and evaluate NEs for the encapsulation of VD3 for the management of vitamin D deficiency in ASD children. Safe ingredients were employed for the preparation of NEs such as vegetable oils and non-ionic surfactants. Safety, on basis of acute and chronic toxicity, and pharmacokinetic studies on experimental animals were performed in comparison with plain VD3. Clinical studies on ASD children are underway.
Materials and Methods
Materials
Vitamin D3 (colecalciferol; VD3) was bought from Sigma-Aldrich Co., USA. 25-Hydroxyvitamin D3 [25(OH)D3] (≥ 97%) and 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) were supplied by Santa Cruz Biotechnology, Inc., USA. Span 20 (Sp20) (extra pure) was purchased from Loba Chemie Pvt. Ltd., India. Glycerol (≥ 99%) was obtained from Fisher Scientific Co., UK. Fructose (99%) was procured from Winlab Ltd., UK. Almond, pumpkin, olive, and wheat germ oils were bought from the specific unit of extracting oils at the National Research Centre (NRC), Cairo, Egypt. The cold press method was used for oil extraction. Mango flavor was purchased from Kamena Co., Egypt. Phosphotungstic acid hydrate was supplied by Fluka analytical, USA. All other chemicals and reagents were of analytical grade. Creatinine, blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), calcium (Ca), and reduced malondialdehyde (MDA) kits were supplied by Biodiagnostic Co., Egypt. Enzyme-linked immunosorbent assay (ELISA) kit of caspase was procured from Sunlong Biotech Co., Ltd., China.
Methods
Preparation of VD3-Loaded NEs
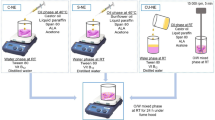
Based on the preliminary studies, Sp20, glycerol, fructose, and mango flavor were selected as the emulsifier, viscosity modifier, sweetening, and flavoring agents, respectively. The preparation of VD3-loaded NE took place by the method previously reported with some modifications [27]. In brief, VD3 (0.0015%, w/v) was dissolved in the vegetable oil (almond, pumpkin, olive or wheat germ oil) (5%, v/v) along with Sp20 (2 or 3%, v/v) and mixed forming the oily phase. Glycerol (10%, v/v), fructose (30%, w/v), mango flavor (0.2%, v/v), and double distilled water were mixed to form the aqueous phase. The oily phase was added slowly to the aqueous phase while mixing on the magnetic stirrer (1500 rpm) for 5 min. Subsequently, the formed mixture was passed across a high-speed homogenizer (SilentCrusher M, Heidolph Instruments GmbH & Co. KG, Germany) at 20,000 rpm for 10 min followed by sonication employing a bath sonicator (Elmasonic S 40 H, Elma Schmidbauer GmbH, Germany) for 15 min. The resulting NE was kept in a refrigerator for upcoming examinations. The composition of the developed VD3-loaded NE formulations is illustrated in Table I.
Characterization of VD3-Loaded NEs
Estimation of VD3 Content
VD3 content in the prepared VD3-loaded NE formulations was determined by liquid chromatography-tandem mass spectrometry (LC–MS/MS). Briefly, 1 ml of the formulation was appropriately diluted by methanol and sonicated in the bath sonicator for 20 min. Afterwards, the solvent was evaporated under a nitrogen stream. The derivatization reagent, namely, 150 μl of PTAD in acetonitrile (1 mg/ml), was added to the residue, vortex-mixed, and kept at room temperature for 1 h for reaction completion. The sample was evaporated under a nitrogen stream followed by reconstitution in 40% acetonitrile (400 μl) to be analyzed [37]. The assessment was carried out in triplicate. VD3 content was computed as follows:
LC–MS/MS was performed on a Waters Xevo TQD triple quadrupole tandem mass spectrometer coupled to Waters Acquity Ultra-Performance Liquid Chromatography (UPLC) system, Waters Co., USA. Chromatographic separation was conducted using Waters Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.9 μm) maintained at 40°C. Acetonitrile (B) and 0.1% formic acid in water (A) were used as mobile phases [37]. Gradient elution was carried out at a flow rate of 0.2 ml/min. The gradient program was as follows: 0 to 3 min 40% B, 9 to 10.5 min 60% B, 11 to 14 min 90% B, and 18 to 25 min 40% B. The injection volume was 5 μl. Mass spectrum (MS) analysis was performed employing a positive mode electrospray ionization method. A multiple reaction mode was used for quantitation with parent daughter mass transition 560.4 < 298.1, cone voltage 30 V, and collision energy 20 V.
Droplet Size, Polydispersity Index, and Zeta Potential Measurements
The droplet size (DS), polydispersity index (PDI), and zeta potential (ZP) of the developed VD3-loaded NE formulations were investigated via dynamic light scattering using a Zetasizer (Nano Series ZS90, Malvern Instruments Ltd., UK) at 25°C. Formulations were appropriately diluted by double distilled water to achieve a suitable scattering intensity. The width of the size distribution was assessed by PDI determination. A high PDI value points to a heterogeneous size distribution, while a low PDI value indicates a greater homogeneity [38].
Employing the same apparatus, ZP of the developed VD3-loaded NE formulations was determined via examining their electrophoretic mobility in an electrical field. All measurements were assessed using 3 independent formulation samples.
pH Measurement
The pH of the prepared VD3-loaded NE formulations was determined using a standard digital pH meter (3510 pH meter, Jenway Co., UK) at 25°C.
Storage Stability Study
The storage stability was inspected by storing the prepared VD3-loaded NE formulations at room (25 ± 2°C) and refrigeration (4 ± 2°C) temperatures for a period of 6 months. Three independent samples from each formulation were kept in firmly sealed glass containers and stored under dark conditions. At 2, 4, and 6 months, samples were visually inspected for any signs of creaming, coalescence, phase separation, and/or precipitation. At the end of the study (6 months), VD3 content, DS, PDI, ZP, and pH of the samples were determined.
Transmission Electron Microscopy (TEM)
Morphological examination of the optimum VD3-loaded NE formulation was performed via TEM (JEM-2100, JEOL Co., Japan). The optimum formulation was appropriately diluted by double distilled water. A drop of the formulation sample was added on a carbon-coated Cu grid and left for 10 min to dry completely. Subsequently, phosphotungstic acid solution (1%, w/v) was added to the sample for staining and air-dried for 10 min at 25°C. Surface characteristics and shape of the optimum NE formulation was then assessed at suitable magnifications.
In vivo Studies
Animals
Healthy male and female Wistar albino rats (120–140 g) were employed for the current studies. Rats were procured from the central animal house at the NRC, Cairo, Egypt. Animals were kept in well-ventilated boxes (22 ± 2°C) on a 12-h light and dark cycle. Rats were fed pelleted food and tap water ad libitum. Rats were humanely treated, and the experiment protocols took place following the ethical guidelines regarding care and use of experimental animals approved by the Medical Research Ethics Committee at the NRC (Reg. No. 19/233).
Acute Toxicity Study
Forty eight Wistar albino rats (24 males and 24 females) were classified into four groups of twelve rats each. Groups were divided in two subgroups containing six rats each, representing both sexes (male and female), and received treatments as follows: group 1 comprised normal control male and female rats receiving a single oral dose of normal saline. Group 2 consisted of male and female rats given a single oral dose of plain VD3 at a dose of 1800 IU/kg, i.e., 45 µg/kg. Male and female rats in groups 3 and 4 were given a single oral dose of the optimum VD3-loaded NE formulation and drug-free NE formulation, respectively, at a dose equivalent to 1800 IU/kg, i.e., 45 µg/kg of VD3. Noteworthy, the administered dose of VD3 was calculated as ten times the recommended daily dose in adults, i.e., 20,000 IU [39], according to conversion tables by Paget and Barnes [40]. Rats were observed daily for a period of 2 weeks. They were checked for occurrence of morbidity and mortality as well as for any change in skin and fur appearance, respiratory rates, central nervous system disturbances (tremors, convulsions, lethargy, sleep disturbance, coma, etc.), and behavioral effects.
Chronic Toxicity Study
Chronic toxicity was carried out according to the World Health Organization research guidelines [41]. Eighty Wistar albino rats (40 males and 40 females) were allocated into four groups of twenty rats each. Groups were divided in two subgroups containing ten rats each of both sexes as follows: group 1 consisted of normal control male and female rats given an oral dose of normal saline once daily for a period of three months. Group 2 comprised male and female rats receiving an oral dose of plain VD3 at a dose of 180 IU/kg, i.e., 4.5 µg/kg once daily for 3 months. Male and female rats in groups 3 and 4 were given a daily oral dose of the optimum VD3-loaded NE formulation and drug-free NE formulation, respectively, at a dose equivalent to 180 IU/kg, i.e., 4.5 µg/kg of VD3 once daily for 3 months. The administered dose of VD3 was calculated according to conversion tables by Paget and Barnes [40] based on a recommended adult daily dose of 2000 IU [39].
At the end of the study (3 months), animals were anesthetized with pentobarbital sodium, and blood samples were taken from the retro-orbital plexus. A complete blood count (CBC) was carried out.
Blood samples were collected then centrifuged at 4°C using a cooling centrifuge (2k15, Sigma Laborzentrifugen GmbH, Germany) at 3000 rpm for 15 min to separate the serum. Creatinine and BUN were determined according to the methods of Bartels et al. [42] and Fawcett and Scott [43], respectively. Liver function enzyme activities (AST and ALT) were estimated in rat serum according to the methods of Reitman and Frankel [44], whereas ALP was assessed according to Belfield and Goldberg [45]. Serum Ca, MDA, and caspase levels were also determined according to Gindler and King [46], Ohkawa et al. [47], and Mohamed et al. [48], respectively.
Pharmacokinetic Study
Twelve male Wistar albino rats were allocated into two groups of six rats each. Group 1 received a single oral dose of plain VD3 at a dose of 180 IU/kg, i.e., 4.5 µg/kg. Group 2 was given a single oral dose of the optimum VD3-loaded NE formulation at a dose equivalent to 180 IU/kg, i.e., 4.5 µg/kg. The administered dose of VD3 is equivalent to the dose used for the chronic toxicity study. At determined time intervals (up to 72 h), blood samples were withdrawn from the retro-orbital venous plexus using heparinized capillary tubes. Blood samples were centrifuged at 3000 rpm for 10 min to separate plasma and kept at − 80°C until LC–MS/MS analysis. The pharmacokinetic parameters for orally administered VD3 were calculated based on a non-compartmental model [49]. The determined parameters were the area under the plasma drug concentration vs. time curve from 0 to 72 h (AUC0–72), maximum plasma drug concentration (Cmax), and time needed to reach maximum plasma drug concentration (Tmax). Parameters were calculated directly from the individual plasma drug concentration vs. time profiles. The data procured from pharmacokinetic parameters were analyzed statistically using PKSolver, an add-in program for pharmacodynamic, and pharmacokinetic data analysis in Microsoft® Excel [50].
After thawing at room temperature, 2 ml of acetonitrile was added to 1 ml of plasma for protein precipitation. Samples were vortex-mixed followed by centrifugation for 10 min at 3000 rpm. The supernatants were transferred to glass tubes, and the volume was reduced to 1 ml by evaporation under nitrogen stream. Vitamin D metabolites were extracted from the remaining solution by liquid–liquid extraction via adding 5 ml of ethyl acetate followed by vigorous shaking for 10 min and then centrifuged for 5 min at 1000 rpm. The upper organic layer was transferred to new glass tubes, and the solvent was completely evaporated under nitrogen stream [37]. Derivatization took place by adding 150 µl of PTAD to the residue and continued as previously discussed in the “Estimation of VD3 Content” section.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was conducted employing one way analysis of variance (ANOVA) followed by Tukey’s post hoc for means comparison using GraphPad Prism software, version 5 (GraphPad Inc., San Diego, USA). The difference was considered significant at p < 0.05.
Results and Discussion
Preparation of VD3-Loaded NEs
All eight NE formulations showed a homogenous white milky appearance. No signs of coalescence or phase separation were reported after 24 h of preparation. The selection of ingredients and their concentrations was mainly focused on safety, taste acceptability, and NE stability. Almond, olive, pumpkin, and wheat germ oils were selected for the present investigation. Vegetable oils are known for their safety on oral administration. A preliminary study was performed to assess the most suitable ingredients and concentrations for the formation of NEs using the four investigated oils. The examined surfactants were Tween 20, Tween 80, and Sp20. NEs prepared employing Tween 20 and Tween 80 revealed cracking after 24 h irrespective of the oil type. On the contrary, NEs prepared employing Sp20 exhibited no signs of separation or cracking with the four oils investigated after 24 h. Using surfactants with hydrophilic–lipophilic balance (HLB) values close to those required for oils results in the formation of more stable emulsions [51, 52]. The required HLB value of vegetable oils is generally around 7 [52]. The required HLB value for the four investigated oils ranges from 6 to 8, making Sp20 (HLB = 8.6) a more appropriate surfactant for NE preparation, compared to Tween 20 and Tween 80 (HLB = 16.72 and 15, respectively). Thus, Sp20 was selected for use in the current study. Sp20 is a non-ionic surfactant commonly employed in cosmetics and pharmaceutical preparations as well as food products. It is considered as a nonirritant and nontoxic ingredient [53, 54]. Glycerol is widely utilized as a sweetening agent, solvent, viscosity-increasing agent, and preservative in oral solutions [54]. Furthermore, the addition of small quantities of glycerol was reported to notably augment the storage stability of oil in water emulsions [55, 56]. Fructose is commonly employed in solutions, syrups, and tablets as a sweetening and flavoring agent. The sweetness-response profile of fructose is recognized more quickly in the mouth compared to that of dextrose and sucrose. This might account for the capability of fructose to improve fruit flavors and hide particular unpleasant mineral or vitamin “off-flavors” [54]. VD3 was incorporated in the NE formulations at a concentration of 15 µg/ml, i.e., 600 IU/ml, which is equivalent to the recommended daily oral dose for children from 1 to 10 years old [39].
Characterization of VD3-Loaded NEs
Estimation of VD3 Content
All developed VD3-loaded NE formulations exhibited high drug content (> 98%), thus confirming that VD3 is completely incorporated in NE formulations (Table II). NEs are found to provide elevated encapsulation efficiency and enhanced drug stability [27].
Droplet Size, Polydispersity Index, and Zeta Potential Measurements
Measurement of DS is a significant parameter for assessing the quality of the prepared NE formulations [57]. Smaller DS results in greater interfacial area for drug absorption leading to quicker absorption and enhanced bioavailability [58]. The DS of the investigated VD3-loaded NE formulations ranged from 61.15 to 129.80 nm indicating that NE droplets were in the nanometric range (Table II). This relatively small DS range is possibly ascribed to the stabilization of oil droplets due to the existence of the surfactant molecules at the oil–water interface [58]. The surfactant might contribute to the condensation and stabilization of the interfacial film, leading to lesser DS [58, 59]. The results also show that formulations containing a higher amount of Sp20 (3%, v/v) exhibited smaller DS compared to their counterparts comprising 2%. This decrease in DS upon increasing the amount of surfactant was previously reported [59,60,61]. Increasing the amount of surfactant efficiently stabilized the droplets via forming a steric barrier on their surface, hence guarding smaller droplets and hindering them to coalesce into bigger ones [27, 62].
PDI reflects the homogeneity of size distribution. The results reveal that PDI values of the tested NE formulations ranged from 0.339 to 0.436 (i.e., < 0.5), which indicates uniformity of DS distribution and good homogeneity [63]. This comes in accordance with previous reports [63, 64]. The results also show that an increase in PDI was observed as the surfactant concentration increased from 2 to 3% (Table II). It has been reported that the concentration of surfactant required to stabilize NEs is considerably high due to the large surface to volume ratio of droplet interfaces. Since many surfactants form micelles at such high concentrations, the continuous phase of NEs usually contains micelles as a reservoir of surfactant for coating the droplet interfaces [65]. Accordingly, it is common to employ surfactants at concentrations higher than their critical micelle concentrations for NE preparation as previously reported [66, 67]. This could plausibly explain the observed increase in the PDI as the surfactant concentration increased.
ZP assessment provides an indication of the electrostatic repulsion between oil droplets. An increase in electrostatic repulsion between droplets decreases the chance of their coalescence into larger globules [57, 68]. The assessed VD3-loaded NE formulations showed moderate negative ZP values ranging from − 9.83 to − 19.22 mV (Table II). The negative charge shown by NE formulations is possibly attributed to the presence of Sp20. Previous reports showed that NE systems, prepared employing non-ionic surfactants, exhibited moderately negative ZP values [69, 70]. This was attributed to adsorption of anionic species from the water such as hydroxyl ions to the droplet surfaces [70]. This would explain the higher |ZP| values recorded for NE formulations consisting of Sp20 (3%, v/v) compared to their corresponding ones containing 2%. Another possible explanation could be the presence of glycerol. Emulsion systems containing glycerol were reported to exhibit negatively charged ZP that increased with the increase of glycerol concentration. This may be explained by its negatively charged hydroxyl side groups [55].
pH Measurement
The pH values of the prepared VD3-loaded NE formulations ranged from 4.59 to 5.89 (Table II). Liquid products possessing a slightly acidic pH have better palatability [71].
Storage Stability Study
NEs are kinetically stable and thermodynamically unstable systems, which will eventually separate into different phases. Hence, they are less sensitive to physical and chemical changes, whereas increasing the temperature promotes the destabilization of NEs because of the change in the dispersed phase solubility and diffusivity [72]. Accordingly, it is essential to assess the stability of NEs under different storage conditions. NEs should maintain their physical and chemical stability during the product’s shelf life [73, 74]. Table III shows the visual inspection of the prepared VD3-loaded NE formulations throughout storage at room (25 ± 2°C) and refrigeration (4 ± 2°C) temperatures for 6 months. Formulation samples stored at room temperature demonstrated coalescence or phase separation after 2, 4, and 6 months. On the other hand, samples stored at refrigeration temperature for 2 months remained stable without any signs of drug precipitation, creaming, coalescence, cracking, or phase separation. Nevertheless, after 4 and 6 months, samples revealed slight creaming, which was resolved after mild shaking (Table III). This observation comes in accordance with a previous study by Desai et al. where all formulations exhibited phase separation at room temperature after 30 days, while no phase separation was observed at refrigerated temperature [75]. Also, cracking was observed in NE systems stored for 50 days at 37°C by Agnish et al., whereas stable systems were observed upon storage at refrigerated temperature [76]. Instability of NE systems at higher temperatures could be attributed to the presence of oxidation products of the oil. Alterations in the characteristics of NE systems stored at room temperature were previously reported by Maruno. This could be justified by a possible chemical instability due to degradation of formulation compounds caused by hydrolysis of the oil, accompanied by greater solubility of specific oil components in the continuous phase [77]. Increased solubility of oil components in the continuous phase would greatly affect physical stability of NE systems, leading to an increase in the rate of Ostwald ripening [78]. These findings indicate that the developed NE formulations as a drug delivery system should be kept at refrigerated conditions throughout the shelf life. Gravitational separation is the process in which NE droplets move upward (creaming) or downward (sedimentation) as a result of having a lower or higher density compared to the surrounding medium. Because most liquid oils have lesser density than water, they will have the tendency to migrate upwards, whereas water will move downwards [79]. Creaming is considered insignificant in NEs until the DS increases to the size of a few microns because of flocculation, coalescence, and Ostwald ripening [72].
At the end of the storage period (6 months), NE formulations stored at refrigeration temperature were checked for their VD3 content, DS, PDI, ZP, and pH. Compared to zero time samples, the change in drug content was non-significant (p > 0.05). Conversely, the results depicted that a significant (p < 0.05) increase in DS was observed compared to zero time samples except for VD3-NE6, where a non-significant difference (p > 0.05) was revealed (Tables II and IV). Furthermore, formulations prepared employing Sp20 (2%, v/v) showed a significant (p < 0.05) increase in DS compared to their counterparts (3%). This finding comes in agreement with previous reports [75, 80]. This could be attributed to the stabilizing effect produced by a higher surfactant concentration. The hydrophobic groups of the surfactant are adsorbed onto the droplet surface, whereas the hydrophilic groups extend into the aqueous phase in the form of a coil, acting as an effective barrier against aggregation [81]. Generally, because of the emulsifier layer adsorbed on the NE droplets, steric interactions will increase the repulsive force which will in turn stabilize the NEs against coalescence and flocculation [72].
Compared to zero time samples, all investigated NE formulations revealed a relative increase in PDI values after 6 months (Tables II and IV). In fact, PDI is potentially correlated to the Ostwald ripening, where a higher PDI indicates a higher difference in chemical potential between droplets [72]. The increase in both DS and PDI of NE systems upon storage was previously reported by Compolo et al. Both parameters increased in all tested formulations in correlation with time of storage up to 28 weeks [69].
On the other hand, a relative decrease in both |ZP| and pH values was observed in all examined NE formulations compared to zero time samples (Tables II and IV). This comes in accordance with previous reports [69, 82,83,84]. This change in ZP values could be attributed to the formation of oxidation products, which cause a reduction in pH of the medium [85]. It was previously reported that NEs, at neutral pH values, exhibit higher stability, whereas at lower pH values, droplets increase in size [86]. Electrostatic repulsion between droplets is diminished at these low pH values as a result of adsorption of hydrogen ions (H+) to the surface of the droplets. This will result in a lower negative charge and aggregation in media of high acidity [87]. It should be noted that the stability of NE systems is owed mainly to steric repulsion rather than electrostatic repulsion [69]. In view of the aforementioned results, it can be obviously seen that NE formulations prepared using olive oil showed a relatively smaller change in DS, ZP, and pH values after 6 month. This could be attributed to the fact that olive oil is less susceptible to oxidation compared to the other vegetable oils because it contains less polyunsaturated fatty acids [52, 88]. Olive oil is characterized by the presence of high fraction of monounsaturated fatty acids [89, 90]. In fact, the higher the degree of unsaturation, the higher the oxidation rate [52]. This is because the hydrogen atom attached to the carbon of the double bond is removed with ease, yielding alkyl radicals [91].
Based on the preceding results, VD3-NE6, exhibiting the smallest DS, optimum PDI, ZP, pH values, and the highest storage stability, was chosen for upcoming examinations.
Transmission Electron Microscopy
Figure 1 shows the TEM micrograph of VD3-NE6. NE droplets appeared as well dispersed, dark stained, and spherical in shape. No aggregation or coalescence was observed. The DS of the NE is in accordance with the hydrodynamic diameter obtained via the dynamic light scattering measurements (the “Droplet Size, Polydispersity Index, and Zeta Potential Measurements” section).
In vivo Studies
Acute Toxicity Study
Daily inspection for 2 weeks of both male and female rats given a single dose of plain VD3, VD3-NE6, and drug-free NE6 formulations did not show any signs of toxicity in both sexes, i.e., no mortality, hair loss, diarrhea, change in skin appearance, or behavioral abnormalities.
Chronic Toxicity Study
The CBC picture of both male and female rats receiving a daily oral dose of plain VD3, VD3-NE6, and drug-free NE6 formulations for 3 months did not demonstrate any significant changes in hematological parameters (p > 0.05) in comparison with the normal control group (Table V).
Oral administration of plain VD3, VD3-NE6, and drug-free NE6 formulations daily for 3 months did not produce any significant changes (p > 0.05) in kidney (creatinine and BUN) and liver (ALT, AST, and ALP) function parameters, oxidative stress (MDA), and apoptosis (caspase) biomarkers as well as serum Ca of both male and female rats compared to the normal control group (Table VI).
In light of the acute and chronic toxicity studies, VD3-NE6 is considered a safe formulation.
Pharmacokinetic Study
The current study was conducted to explore the ability of VD3-NE6 to improve the gastrointestinal absorption of VD3 after oral administration. Determination of serum 25(OH)D3 metabolite is usually accepted as the most reliable indicator for evaluation of the individual VD3 blood level [49]. The plasma 25(OH)D3 concentration vs. time curve for both plain VD3 and VD3-NE6 is presented in Fig. 2. An obvious improvement of pharmacokinetic parameters (AUC, Cmax, and Tmax) in rats given a single oral dose of VD3-NE6 compared to plain VD3 group was observed. Determination of the pharmacokinetic parameters is important for comparative bioavailability studies. AUC represents the extent of drug absorption, while Cmax and Tmax are essential parameters of the plasma drug level profile, which are characteristic parameters of the drug formulation [92]. Evaluation of the pharmacokinetic parameters showed a significant increase (p < 0.05) in AUC0–72 (316.18 ng.h/ml) and Cmax (8.01 ng/ml) of rats receiving VD3-NE6 compared to plain VD3 (173.05 ng.h/ml and 4.69 ng/ml) by 1.8-fold and 1.7-fold, respectively (Table VII). Additionally, VD3-NE6 group revealed a clear decrease in Tmax (4 h) compared to plain VD3 group (7.33 h). These results indicate the superiority of VD3-NE6 in increasing the absorption of VD3. This comes in agreement with a previous study [49].
VD3 is known to have poor water solubility and oral bioavailability (less than 50%) [22]. It is generally absorbed at definite sites in the small intestine together with fatty acids and fats by means of active and passive transport [23]. NEs have been previously reported to increase oral bioavailability of lipophilic drugs [75, 93]. The surfactant component of the NE may increase permeability through direct partitioning into the cell membrane and disrupting the structural organization of the lipid bilayer resulting in penetration improvement [75]. Furthermore, the lipophilic components in the NE have strong affinity with the lymphatic system and thereby may increase drug transport through the intestinal lymphatic pathway. Having the drug in a solubilized form within the NE might also be an important factor [93]. Additionally, VD3-NE6 exhibited a comparatively small DS (< 100 nm) which plays an important role in NE stability and a crucial step in the course of improving drug bioavailability [94, 95]. Smaller DS results in a greater interfacial area for drug absorption leading to quicker absorption and enhanced bioavailability [58]. Moreover, nanocarrier systems are reported to extend residence time on mucosal membranes with permeation enhancing properties leading to an increased drug uptake [96]. Other reports demonstrated that the nanometric size of emulsion droplets enhanced intestinal permeation of therapeutic agents [97, 98]. Accordingly, the developed VD3-NE6 formulation would potentially result in improved VD3 oral bioavailability [24, 25].
In light of the obtained results, VD3-NE6 would be a good candidate for VD3 supplementation for autistic children. It has been previously reported that vitamin D levels are significantly lower in children with ASD compared to healthy controls, which could classify them as vitamin D inadequate. Accordingly, it is suggested that vitamin D insufficiency may play a role in the etiology of autism [20]. Previous studies have assessed the effect of VD3 supplementation in children with ASD. However, inconsistent results were reported as VD3 supplementation significantly improved the outcome of some children with ASD [99]. Conversly, other reports revealed limited and inconsistent effects of VD3 supplementation [100]. The clinical study to be undertaken by the authors aims to further investigate the subject through making use of the potential enhancement of VD3 bioavailability of VD3-NE6. Supplementation with VD3-NE6 will be provided to children with ASD and compared to a marketed product containing an equivalent dose of VD3. VD3-NE6 is expected to overcome poor intestinal absorption peculiar to autistic children. This will potentially lead to improving vitamin D blood levels and better management of the disease.
Conclusion
Successful preparation of eight VD3-loaded NE formulations was achieved employing four different vegetable oils (5%), Sp20 (2 and 3%), glycerol (10%), fructose (30%), and mango flavor (0.2%). All formulations have efficiently encapsulated VD3 (> 98%) with nanometric DS (61.15–129.8 nm), narrow size distributions (< 0.5), moderately negative ZP (− 9.83 to − 19.22 mV), and acceptable pH values (4.59–5.89). The storage stability study revealed that all formulations exhibited coalescence and phase separation at room temperature after 6 months. At refrigerated conditions, all formulations showed no signs of physical instability for 2 months and only slight creaming after 4 and 6 months. The optimum formulation, VD3-NE6, comprising olive oil (5%) and Sp20 (3%), exhibited a non-significant DS growth upon storage at refrigerated temperature. It appeared as spherical droplets under TEM with no aggregations. Toxicological assessments in male and female rats for 3 months proved the safety of the optimum formulation. Compared to normal control animals, no significant changes to blood picture, kidney, and liver functions as well as serum Ca, oxidative stress, and apoptosis biomarkers were reported. Moreover, a single oral dose of VD3-NE6 given to male rats demonstrated improved pharmacokinetic parameters in comparison with plain VD3, indicating a better drug absorption. Hence, VD3-NE6 depicted a great potential for oral delivery of VD3 with enhanced stability, safety, and bioavailability. Meanwhile, further studies on the clinical management of vitamin D inadequacy in children with ASD are underway.
Data Availability
All data generated or analysed during this study will be available on reasonable request.
References
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011;11:111. https://doi.org/10.1186/1471-2431-11-111.
Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiol. 2011;22(4):476–85. https://doi.org/10.1097/EDE.0b013e31821d0e30.
Bjorklund G, Waly MI, Al-Farsi Y, Saad K, Dadar M, Rahman MM, et al. The role of vitamins in autism spectrum disorder: what do we know? J Mol Neurosci. 2019;67(3):373–87. https://doi.org/10.1007/s12031-018-1237-5.
Petitpierre G, Luisier A-C, Bensafi M. Eating behavior in autism: senses as a window towards food acceptance. Curr Opin Food Sci. 2021;41:210–6. https://doi.org/10.1016/j.cofs.2021.04.015.
Chen N, Watanabe K, Kobayakawa T, Wada M. Relationships between autistic traits, taste preference, taste perception, and eating behaviour. Eur Eat Disord Rev. 2022;30(5):628–40. https://doi.org/10.1002/erv.2931.
Robea MA, Luca AC, Ciobica A. Relationship between vitamin deficiencies and co-occurring symptoms in autism spectrum disorder. Medicina. 2020;56(5). https://doi.org/10.3390/medicina56050245.
Kaluzna-Czaplinska J, Michalska M, Rynkowski J. Vitamin supplementation reduces the level of homocysteine in the urine of autistic children. Nutr Res. 2011;31(4):318–21. https://doi.org/10.1016/j.nutres.2011.03.009.
Schreck KA. Autism, parents, and treatments for their children. In: Patel VB, Preedy VR, Martin CR, editors. Comprehensive Guide to Autism. New York: Springer; 2014. p. 2283–96.
Moulas AN, Vaiou M. Vitamin D fortification of foods and prospective health outcomes. J Biotechnol. 2018;285:91–101. https://doi.org/10.1016/j.jbiotec.2018.08.010.
Pike JW, Christakos S. Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin North Am. 2017;46(4):815–43. https://doi.org/10.1016/j.ecl.2017.07.001.
Marwaha RK, Tandon N, Reddy DRH, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005;82(2):477–82. https://doi.org/10.1093/ajcn/82.2.477.
Harinarayan CV, Joshi SR. Vitamin D status in India–its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8.
Maurya VK, Aggarwal M. A phase inversion based nanoemulsion fabrication process to encapsulate vitamin D3 for food applications. J Steroid Biochem Mol Biol. 2019;190:88–98. https://doi.org/10.1016/j.jsbmb.2019.03.021.
Luo Y, Teng Z, Wang Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem. 2012;60(3):836–43. https://doi.org/10.1021/jf204194z.
Wilson LR, Tripkovic L, Hart K, Smith CP, Bucca G, Elliott R, et al. Mechanisms for differences in the efficacy of vitamin D2 and vitamin D3: assessment of post-supplementation decline in vitamin D status in the D2–D3 Study. Proc Nutr Soc. 2016;75(OCE3):E116. https://doi.org/10.1017/S0029665116001312.
Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115–24. https://doi.org/10.2340/00015555-0980.
Cannell JJ. Vitamin D and autism, what’s new? Rev Endocr Metab Disord. 2017;18(2):183–93. https://doi.org/10.1007/s11154-017-9409-0.
Kocovska E, Fernell E, Billstedt E, Minnis H, Gillberg C. Vitamin D and autism: clinical review. Res Dev Disabil. 2012;33(5):1541–50. https://doi.org/10.1016/j.ridd.2012.02.015.
Vinkhuyzen AAE, Eyles DW, Burne THJ, Blanken LME, Kruithof CJ, Verhulst F, et al. Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open. 2017;3(2):85–90. https://doi.org/10.1192/bjpo.bp.116.004077.
Abdel Meguid N, Hashish AF, Anwar M, Sidhom G. Reduced serum levels of 25-hydroxy and 1,25-dihydroxy vitamin D in Egyptian children with autism. J Altern Complement Med. 2010;16(6):641–5. https://doi.org/10.1089/acm.2009.0349.
Jia F, Wang B, Shan L, Xu Z, Staal WG, Du L. Core symptoms of autism improved after vitamin D supplementation. Pediatrics. 2015;135(1):e196–8. https://doi.org/10.1542/peds.2014-2121.
Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoecklin E, Goessl R, et al. Pharmacokinetics of oral vitamin D3 and calcifediol. Bone. 2014;59:14–9. https://doi.org/10.1016/j.bone.2013.10.014.
Goncalves A, Roi S, Nowicki M, Dhaussy A, Huertas A, Amiot MJ, et al. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 2015;172:155–60. https://doi.org/10.1016/j.foodchem.2014.09.021.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48. https://doi.org/10.1038/nrd2197.
Reboul E, Goncalves A, Comera C, Bott R, Nowicki M, Landrier JF, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol Nutr Food Res. 2011;55(5):691–702. https://doi.org/10.1002/mnfr.201000553
Kassem AA. Nanotechnology inspired advanced engineering fundamentals for optimizing drug delivery. Curr Drug Targets. 2018;19(15):1839–54. https://doi.org/10.2174/1389450119666180207092831.
Kassem AA, Salama A, Mohsen AM. Formulation and optimization of cationic nanoemulsions for enhanced ocular delivery of dorzolamide hydrochloride using Box-Behnken design: in vitro and in vivo assessments. J Drug Deliv Sci Tech. 2022;68:103047. https://doi.org/10.1016/j.jddst.2021.103047.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. https://doi.org/10.1016/j.jconrel.2017.03.008.
Mostafa DM, Abd El-Alim SH, Kassem AA. Nanoemulsions: a new approach for enhancing phytonutrient efficacy. In: Oprea AE, Grumezescu AM, editors. Nanotechnology Applications in Food. Academic Press; 2017. p. 107–27.
Santos-Magalhaes NS, Pontes A, Pereira VM, Caetano MN. Colloidal carriers for benzathine penicillin G: nanoemulsions and nanocapsules. Int J Pharm. 2000;208(1–2):71–80. https://doi.org/10.1016/S0378-5173(00)00546-9.
Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–109:303–18. https://doi.org/10.1016/j.cis.2003.10.023.
Brusewitz C, Schendler A, Funke A, Wagner T, Lipp R. Novel poloxamer-based nanoemulsions to enhance the intestinal absorption of active compounds. Int J Pharm. 2007;329(1–2):173–81. https://doi.org/10.1016/j.ijpharm.2006.08.022.
Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Interface Sci. 2009;14(1):3–15. https://doi.org/10.1016/j.cocis.2008.01.002.
Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75(1):R50–7. https://doi.org/10.1111/j.1750-3841.2009.01457.x.
Hatanaka J, Chikamori H, Sato H, Uchida S, Debari K, Onoue S, et al. Physicochemical and pharmacological characterization of alpha-tocopherol-loaded nano-emulsion system. Int J Pharm. 2010;396(1–2):188–93. https://doi.org/10.1016/j.ijpharm.2010.06.017.
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–10. https://doi.org/10.1016/j.cocis.2005.06.004.
Wang Z, Senn T, Kalhorn T, Zheng XE, Zheng S, Davis CL, et al. Simultaneous measurement of plasma vitamin D3 metabolites, including 4β,25-dihydroxyvitamin D3, using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418(1):126–33. https://doi.org/10.1016/j.ab.2011.06.043.
Asfour MH, Salama AAA, Mohsen AM. Fabrication of all-trans retinoic acid loaded chitosan/tripolyphosphate lipid hybrid nanoparticles as a novel oral delivery approach for management of diabetic nephropathy in rats. J Pharm Sci. 2021;110(9):3208–20. https://doi.org/10.1016/j.xphs.2021.05.007.
Rusińska A, Płudowski P, Walczak M, Borszewska-Kornacka MK, Bossowski A, Chlebna-Sokół D, et al. Vitamin D supplementation guidelines for general population and groups at risk of vitamin d deficiency in Poland—recommendations of the polish society of pediatric endocrinology and diabetes and the expert panel with participation of national specialist consultants and representatives of scientific societies—2018 Update. Front Endocrinol. 2018;9:246. https://doi.org/10.3389/fendo.2018.00246.
Paget GE, Barnes JM. Toxicity Test. In: Laurence DR, Bacharach AL, editors. Evaluation of drug activities. London, New York: Academic Press; 1964. p. 135–66.
World Health Organization. Research guidelines for evaluating the safety and efficacy of herbal medicines. Manila: WHO Regional Office for the Western Pacific; 1993.
Bartels H, Bohmer M, Heierli C. Serum creatinine determination without protein precipitation. Clin Chim Acta. 1972;37:193–7.
Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13(2):156–9. https://doi.org/10.1136/jcp.13.2.156.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. https://doi.org/10.1093/ajcp/28.1.56.
Belfield A, Goldberg DM. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12(5):561–73.
Gindler EM, King JD. Rapid colorimetric determination of calcium in biologic fluids with methylthymol blue. Am J Clin Pathol. 1972;58(4):376–82. https://doi.org/10.1093/ajcp/58.5.376.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. https://doi.org/10.1016/0003-2697(79)90738-3.
Mohamed AL, Elmotasem H, Salama AAA. Colchicine mesoporous silica nanoparticles/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int J Biol Macromol. 2020;164:1149–63. https://doi.org/10.1016/j.ijbiomac.2020.07.133.
Wei-hong T, Min-chang G, Zhen X, Jie S. Pharmacological and pharmacokinetic studies with vitamin D-loaded nanoemulsions in asthma model. Inflammation. 2014;37(3):723–8. https://doi.org/10.1007/s10753-013-9790-0.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–14. https://doi.org/10.1016/j.cmpb.2010.01.007.
Hong IK, Kim SI, Lee SB. Effects of HLB value on oil-in-water emulsions: droplet size, rheological behavior, zeta-potential, and creaming index. J Ind Eng Chem. 2018;67:123–31. https://doi.org/10.1016/j.jiec.2018.06.022.
Kampa J, Frazier R, Rodriguez-Garcia J. Physical and chemical characterisation of conventional and nano/emulsions: influence of vegetable oils from different origins. Foods. 2022;11(5):681. https://doi.org/10.3390/foods11050681.
Partridge D, Lloyd KA, Rhodes JM, Walker AW, Johnstone AM, Campbell BJ. Food additives: assessing the impact of exposure to permitted emulsifiers on bowel and metabolic health – introducing the FADiets study. Nutr Bull. 2019;44(4):329–49. https://doi.org/10.1111/nbu.12408.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6th ed. London, Chicago: Pharmaceutical Press and the American Pharmacists Association; 2009.
Mirhosseini H, Tan CP, Taherian AR. Effect of glycerol and vegetable oil on physicochemical properties of Arabic gum-based beverage emulsion. Eur Food Res Technol. 2008;228(1):19–28. https://doi.org/10.1007/s00217-008-0901-3.
Ayannides CA, Ktistis G. Stability estimation of emulsions of isopropyl myristate in mixtures of water and glycerol. J Cosmet Sci. 2002;53(3):165–73.
Kassem AA, Abd El-Alim SH, Salman AM, Mohammed MA, Hassan NS, El-Gengaihi SE. Improved hepatoprotective activity of Beta vulgaris L. leaf extract loaded self-nanoemulsifying drug delivery system (SNEDDS): in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2020;46(10):1589–603. https://doi.org/10.1080/03639045.2020.1811303.
Kassem AA, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: design, optimization, in vitro and in vivo evaluation. J Mol Liq. 2016;218:219–32. https://doi.org/10.1016/j.molliq.2016.02.081.
Hosny KM, Banjar ZM. The formulation of a nasal nanoemulsion zaleplon in situ gel for the treatment of insomnia. Expert Opin Drug Deliv. 2013;10(8):1033–41. https://doi.org/10.1517/17425247.2013.812069.
Guttoff M, Saberi AH, McClements DJ. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem. 2015;171:117–22. https://doi.org/10.1016/j.foodchem.2014.08.087.
Mostafa DM, Abd El-Alim SH, Asfour MH, Al-Okbi SY, Mohamed DA, Hamed TE, et al. Transdermal fennel essential oil nanoemulsions with promising hepatic dysfunction healing effect: in vitro and in vivo study. Pharm Dev Technol. 2019;24(6):729–38. https://doi.org/10.1080/10837450.2019.1584633.
Rahman Z, Zidan AS, Khan MA. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm. 2010;76(1):127–37. https://doi.org/10.1016/j.ejpb.2010.05.003.
Jusril NA, Abu Bakar SI, Khalil KA, Md Saad WM, Wen NK, Adenan MI. Development and optimization of nanoemulsion from ethanolic extract of Centella asiatica (NanoSECA) using D-optimal mixture design to improve blood-brain barrier permeability. Evid Based Complement Alternat Med. 2022;2022:3483511. https://doi.org/10.1155/2022/3483511.
Yalcin TE, Takka S. Development and characterization of camphor-loaded ozonated olive oil nanoemulsions. J Res Pharm (online). 2020;24(6):935–42. https://doi.org/10.35333/jrp.2020.253
Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter. 2006;18(41):R635. https://doi.org/10.1088/0953-8984/18/41/R01.
Sarheed O, Dibi M, Ramesh K. Studies on the effect of oil and surfactant on the formation of alginate-based O/W lidocaine nanocarriers using nanoemulsion template. Pharmaceutics. 2020;12(12). https://doi.org/10.3390/pharmaceutics12121223.
Zeng L, Liu Y, Yuan Z, Wang Z. Formation and physical stability of Zanthoxylum bungeanum essential oil based nanoemulsions co-stabilized with tea saponin and synthetic surfactant. Molecules. 2021;26(24). https://doi.org/10.3390/molecules26247464.
Wang H, Li Q, Deng W, Omari-Siaw E, Wang Q, Wang S, et al. Self-nanoemulsifying drug delivery system of trans-cinnamic acid: formulation development and pharmacodynamic evaluation in alloxan-induced type 2 diabetic rat model. Drug Dev Res. 2015;76(2):82–93. https://doi.org/10.1002/ddr.21244.
Campolo O, Giunti G, Laigle M, Michel T, Palmeri V. Essential oil-based nano-emulsions: effect of different surfactants, sonication and plant species on physicochemical characteristics. Industrial Crops and Products. 2020;157:112935. https://doi.org/10.1016/j.indcrop.2020.112935.
Ziani K, Chang Y, McLandsborough L, McClements DJ. Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. J Agric Food Chem. 2011;59(11):6247–55. https://doi.org/10.1021/jf200450m.
Mohapatra A, Parikh RK, Gohel MC. Formulation, development and evaluation of patient friendly dosage forms of metformin, Part-III: soluble effervescent tablets. Asian J Pharm. 2008;2(3):177–81.
Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–41. https://doi.org/10.1039/C5SM02958A.
Galvão KCS, Vicente AA, Sobral PJA. Development, characterization, and stability of o/w pepper nanoemulsions produced by high-pressure homogenization. Food Bioproc Tech. 2018;11(2):355–67. https://doi.org/10.1007/s11947-017-2016-y.
Nejadmansouri M, Hosseini SMH, Niakosari M, Yousefi GH, Golmakani MT. Physicochemical properties and storage stability of ultrasound-mediated WPI-stabilized fish oil nanoemulsions. Food Hydrocoll. 2016;61:801–11. https://doi.org/10.1016/j.foodhyd.2016.07.011.
Desai J, Thakkar H. Enhanced oral bioavailability and brain uptake of Darunavir using lipid nanoemulsion formulation. Colloids Surf B Biointerfaces. 2019;175:143–9. https://doi.org/10.1016/j.colsurfb.2018.11.057.
Agnish S, Sharma AD, Kaur I. Nanoemulsions (O/W) containing Cymbopogon pendulus essential oil: development, characterization, stability study, and evaluation of in vitro anti-bacterial, anti-inflammatory, anti-diabetic activities. BioNanoScience. 2022;12(2):540–54. https://doi.org/10.1007/s12668-022-00964-4.
Maruno M. Characterization and stability studies on vegetable nanoemulsions obtained by low energy process. J Nanomed Nanotechnol. 2017;2017:1–8. https://doi.org/10.4172/2157-7439.S8-003.
Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and ostwald ripening stability. Langmuir. 2008;24(22):12758–65. https://doi.org/10.1021/la801685v.
Liu Q, Huang H, Chen H, Lin J, Wang Q. Food-grade nanoemulsions: preparation, stability and application in encapsulation of bioactive compounds. Molecules. 2019;24(23):4242. https://doi.org/10.3390/molecules24234242.
Liu W, Sun D, Li C, Liu Q, Xu J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J Colloid Interface Sci. 2006;303(2):557–63. https://doi.org/10.1016/j.jcis.2006.07.055.
Kim DW, Shin SI, Oh SG. Preparation and stabilization of silver colloids in aqueous surfactant solutions. In: Mittal KL, Shah DO, editors. Adsorption and Aggregation of Surfactants in Solution. New York: Marcel Dekker, Inc.; 2003. p. 255–68.
Ali A, Rehman A, Shehzad Q, Khan S, Karim A, Afzal N, et al. Development and characterization of nanoemulsions incorporating tuna fish oil. Int J Res Agric Sci. 2020;7(1):2348–3997.
Shehzad Q, Rehman A, Ali A, Khan S, Mahdi AA, Karim A, et al. Preparation and characterization of resveratrol loaded nanoemulsions. Int J Agric Innov Res. 2020;8(4):300–10.
Chuacharoen T, Prasongsuk S, Sabliov CM. Effect of surfactant concentrations on physicochemical properties and functionality of curcumin nanoemulsions under conditions relevant to commercial utilization. Molecules. 2019;24(15). https://doi.org/10.3390/molecules24152744.
Polavarapu S, Oliver CM, Ajlouni S, Augustin MA. Physicochemical characterisation and oxidative stability of fish oil and fish oil–extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 2011;127(4):1694–705. https://doi.org/10.1016/j.foodchem.2011.02.044.
Rao J, McClements DJ. Formation of flavor oil microemulsions, nanoemulsions and emulsions: influence of composition and preparation method. J Agric Food Chem. 2011;59(9):5026–35. https://doi.org/10.1021/jf200094m.
Chuah AM, Kuroiwa T, Kobayashi I, Nakajima M. Effect of chitosan on the stability and properties of modified lecithin stabilized oil-in-water monodisperse emulsion prepared by microchannel emulsification. Food Hydrocoll. 2009;23(3):600–10. https://doi.org/10.1016/j.foodhyd.2008.03.014.
Kiokias SN, Dimakou CP, Tsaprouni IV, Oreopoulou V. Effect of compositional factors against the thermal oxidative deterioration of novel food emulsions. Food Biophys. 2006;1(3):115. https://doi.org/10.1007/s11483-006-9015-2.
Jimenez-Lopez C, Carpena M, Lourenco-Lopes C, Gallardo-Gomez M, Lorenzo JM, Barba FJ, et al. Bioactive compounds and quality of extra virgin olive oil. Foods. 2020;9(8):1014. https://doi.org/10.3390/foods9081014.
Al-Bachir M, Sahloul H. Fatty acid profile of olive oil extracted from irradiated and non-irradiated olive fruits. Int J Food Prop. 2017;20(11):2550–8. https://doi.org/10.1080/10942912.2016.1243557.
Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf. 2006;5(4):169–86. https://doi.org/10.1111/j.1541-4337.2006.00009.x.
Liu J, Shentu JZ, Wu LH, Dou J, Xu QY, Zhou HL, et al. Relative bioavailability and pharmacokinetic comparison of two different enteric formulations of omeprazole. J Zhejiang Univ Sci B. 2012;13(5):348–55. https://doi.org/10.1631/jzus.B1100272.
Zeng F, Wang D, Tian Y, Wang M, Liu R, Xia Z, et al. Nanoemulsion for improving the oral bioavailability of hesperetin: formulation optimization and absorption mechanism. J Pharm Sci. 2021;110(6):2555–61. https://doi.org/10.1016/j.xphs.2021.02.030.
Balakumar K, Raghavan CV, selvan NT, prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–43. https://doi.org/10.1016/j.colsurfb.2013.08.025.
Xi J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10(1):172–82. https://doi.org/10.1208/s12249-009-9190-9.
Bernkop-Schnurch A. Nanocarrier systems for oral drug delivery: do we really need them? Eur J Pharm Sci. 2013;49(2):272–7. https://doi.org/10.1016/j.ejps.2013.03.008.
Asfour MH, Kassem AA, Salama A, Abd El-Alim SH. Hydrophobic ion pair loaded self-emulsifying drug delivery system (SEDDS): a novel oral drug delivery approach of cromolyn sodium for management of bronchial asthma. Int J Pharm. 2020;585:119494. https://doi.org/10.1016/j.ijpharm.2020.119494.
Phan TNQ, Shahzadi I, Bernkop-Schnurch A. Hydrophobic ion-pairs and lipid-based nanocarrier systems: the perfect match for delivery of BCS class 3 drugs. J Control Release. 2019;304:146–55. https://doi.org/10.1016/j.jconrel.2019.05.011.
Feng J, Shan L, Du L, Wang B, Li H, Wang W, et al. Clinical improvement following vitamin D3 supplementation in autism spectrum disorder. Nutr Neurosci. 2017;20(5):284–90. https://doi.org/10.1080/1028415X.2015.1123847.
Kerley CP, Power C, Gallagher L, Coghlan D. Lack of effect of vitamin D3 supplementation in autism: a 20-week, placebo-controlled RCT. Arch Dis Child. 2017;102(11):1030–6. https://doi.org/10.1136/archdischild-2017-312783.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The present study was funded by the project’s sector at the National Research Centre (NRC), Cairo, Egypt (research group project fund no. 12060135).
Author information
Authors and Affiliations
Contributions
Marwa Hasanein Asfour: conceptualization, methodology, investigation, data curation, writing original draft, and writing review and editing.
Sameh Hosam Abd El-Alim: conceptualization, methodology, data curation, investigation, writing original draft, and writing review and editing.
Ahmed Alaa Kassem: conceptualization, methodology, data curation, investigation, writing original draft, and writing review and editing.
Abeer Salama: methodology, investigation, data curation, and writing original draft.
Amr Sobhi Gouda: methodology, validation, and investigation.
Walaa Samy Nazim: methodology, validation, and investigation.
Neveen Hassan Nashaat: resources and software.
Maha Hemimi: resources and software.
Nagwa Abdel Meguid: conceptualization, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asfour, M.H., Abd El-Alim, S.H., Kassem, A.A. et al. Vitamin D3-Loaded Nanoemulsions as a Potential Drug Delivery System for Autistic Children: Formulation Development, Safety, and Pharmacokinetic Studies. AAPS PharmSciTech 24, 58 (2023). https://doi.org/10.1208/s12249-023-02501-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02501-2