Abstract
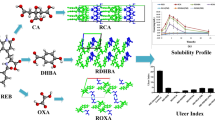
Co-crystallization studies were undertaken to improve the solubility of a highly water-insoluble drug febuxostat (FXT), used in the treatment of gout and hyperuricemia. The selection of co-crystal former (CCF) molecules such as 1-hydroxy 2-naphthoic acid (1H-2NPH), 4-hydroxy benzoic acid (4-HBA), salicylic acid (SAC), 5-nitro isophthalic acid (5-NPH), isonicotinamide (ISNCT), and picolinamide (PICO) was based on the presence of complementary functional groups capable of forming hydrogen bond and the ΔpKa difference between FXT and CCF. A liquid-assisted grinding (LAG) method was successfully employed for the rapid screening of various pharmaceutical adducts. These adducts were characterized based on their unique thermal (differential scanning calorimetry) and spectroscopic (Fourier transform infrared and Raman spectroscopy) profiles. Binary phase diagrams (BPD) were plotted to establish a relationship between the thermal events and adduct formed. Powder X-ray diffraction (PXRD) studies were carried out to confirm the formation of eutectic/co-crystal. Thermogravimetric analysis (TGA) was also performed for the novel co-crystals obtained. The propensity for strong homo-synthons over weak hetero-synthons and strong hetero-synthons over weak homo-synthons during supramolecular growth resulted in the formation of eutectics and co-crystals respectively. FXT:1H-2NPH (1), FXT:4-HBA (1), FXT:SAC (1, 2), and FXT:5-NPH (2-1) gave rise to pure eutectic systems, while FXT:ISNCT (2-1) and FXT:PICO (1) gave rise to novel co-crystals with characteristic DSC heating curves and PXRD pattern. Additionally, the impact of microenvironmental pH and microspeciation profile on the improved dissolution profile of the co-crystals was discussed.

Graphical Abstract













Similar content being viewed by others
References
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:1–10.
Stahl PH. Handbook of pharmaceutical salts properties, selection, and use. In: Stahl PH, Wermuth CG, editors. International Union of Pure and Applied Chemistry. John Wiley & Sons; 2008. p. 374.
Sun CC. Cocrystallization for successful drug delivery. Expert Opin Drug Deliv. 2013;10(2):201–13.
Vishweshwar P, McMahon JA, Bis JA, Zaworotko MJ. Pharmaceutical co-crystals. J Pharm Sci. 2006;95(3):499–516.
Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9(6):2950–67.
Good DJ, Rodríguez-Hornedo N. Solubility advantage of pharmaceutical cocrystals. Cryst Growth Des. 2009;9(5):2252–64.
Duggirala NK, Perry ML, Almarsson Ö, Zaworotko MJ. Pharmaceutical cocrystals: along the path to improved medicines. Chem Commun. 2016;52(4):640–55.
Remenar JF, Morissette SL, Peterson ML, Moulton B, MacPhee JM, Guzmán HR, et al. Crystal engineering of novel cocrystals of a triazole drug with 1, 4-dicarboxylic acids. J Am Chem Soc. 2003;125(28):8456–7.
Jung MS, Kim JS, Kim MS, Alhalaweh A, Cho W, Hwang SJ, Velaga SP. Bioavailability of indomethacin-saccharin cocrystals. J Pharm Pharmacol. 2010;62(11):1560–8.
Rahman Z, Agarabi C, Zidan AS, Khan SR, Khan MA. Physico-mechanical and stability evaluation of carbamazepine cocrystal with nicotinamide. AAPS PharmSciTech. 2011;12(2):693–704.
Karki S, Friščić T, Fábián L, Laity PR, Day GM, Jones W. Improving mechanical properties of crystalline solids by cocrystal formation: new compressible forms of paracetamol. Adv Mater. 2009;21(38-39):3905–9.
Trask AV, Motherwell WS, Jones W. Physical stability enhancement of theophylline via cocrystallization. Int J Pharm. 2006;320(1):114–23.
Blagden N, Coles S, Berry D. Pharmaceutical co-crystals–are we there yet? CrystEngComm. 2014;16(26):5753–61.
Kumar A, Kumar S, Nanda A. A review about regulatory status and recent patents of pharmaceutical co-crystals. Adv Pharm Bull. 2018;8(3):355–63.
Cherukuvada S, Nangia A. Eutectics as improved pharmaceutical materials: design, properties and characterization. Chem Commun. 2014;50(8):906–23.
Kang Y, Gu J, Hu X. Syntheses, structure characterization and dissolution of two novel cocrystals of febuxostat. J Mol Struct. 2017;1130:480–6.
Maddileti D, Jayabun S, Nangia A. Soluble cocrystals of the xanthine oxidase inhibitor febuxostat. Cryst Growth Des. 2013;13(7):3188–96.
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm. 2013;453(1):101–25.
Etter MC. Hydrogen bonds as design elements in organic chemistry. J Phys Chem. 1991;95(12):4601–10.
Childs SL, Stahly GP, Park A. The salt-cocrystal continuum: the influence of crystal structure on ionization state. Mol Pharm. 2007;4(3):323–38.
Childs SL, Rodríguez-Hornedo N, Reddy LS, Jayasankar A, Maheshwari C, McCausland L, Shipplett R, Stahly BC. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. CrystEngComm. 2008;10(7):856–64.
Friščić T, Childs SL, Rizvi SA, Jones W. The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm. 2009;11(3):418–26.
Toda F, Braga D, editors. Organic solid state reactions. In: Volume 254 of topics in current chemistry. Springer Science & Business Media; 2005. pp. 313
Stoler E, Warner JC. Non-covalent derivatives: cocrystals and eutectics. Molecules. 2015;20(8):14833–48.
Leung DH, Lohani S, Ball RG, Canfield N, Wang Y, Rhodes T, Bak A. Two novel pharmaceutical cocrystals of a development compound–screening, scale-up, and characterization. Cryst Growth Des. 2012;12(3):1254–62.
Sekhon B. Pharmaceutical co-crystals-a review. Ars Pharm. 2009;50(3):99–117.
Cruz-Cabeza AJ. Acid–base crystalline complexes and the p K a rule. CrystEngComm. 2012;14(20):6362–5.
Patel J, Jagia M, Bansal AK, Patel S. Characterization and thermodynamic relationship of three polymorphs of a xanthine oxidase inhibitor, febuxostat. J Pharm Sci. 2015;104(11):3722–30.
Badawy SIF, Hussain MA. Microenvironmental pH modulation in solid dosage forms. J Pharm Sci. 2007;96(5):948–59.
Lawrence XY, Carlin AS, Amidon GL, Hussain AS. Feasibility studies of utilizing disk intrinsic dissolution rate to classify drugs. Int J Pharm. 2004;270(1):221–7.
Issa MG, Ferraz HG. Intrinsic dissolution as a tool for evaluating drug solubility in accordance with the biopharmaceutics classification system. Dissolut Technol. 2011;18(3):6–11.
Lu E, Rodríguez-Hornedo N, Suryanarayanan R. A rapid thermal method for cocrystal screening. CrystEngComm. 2008;10(6):665–8.
Cherukuvada S, Guru Row TN. Comprehending the formation of eutectics and cocrystals in terms of design and their structural interrelationships. Cryst Growth Des. 2014;14(8):4187–98.
Yamashita H, Hirakura Y, Yuda M, Terada K. Coformer screening using thermal analysis based on binary phase diagrams. Pharm Res. 2014;31(8):1946–57.
Yamashita H, Hirakura Y, Yuda M, Teramura T, Terada K. Detection of cocrystal formation based on binary phase diagrams using thermal analysis. Pharm Res. 2013;30(1):70–80.
Pal S, Roopa B, Abu K, Manjunath SG, Nambiar S. Thermal studies of furosemide–caffeine binary system that forms a cocrystal. J Therm Anal Calorim. 2014;115(3):2261–8.
Félix-Sonda BC. Rivera-Islas Js, Herrera-Ruiz D, Morales-Rojas H, Höpfl H. Nitazoxanide cocrystals in combination with succinic, glutaric, and 2, 5-dihydroxybenzoic acid. Cryst Growth Des. 2014;14(3):1086–102.
Stuart BH. Infrared spectroscopy: fundamentals and applications. In: Analytical Techniques in the Sciences (AnTs). John Wiley & Sons; 2004. pp. 248.
Bakiler M, Bolukbasi O, Yilmaz A. An experimental and theoretical study of vibrational spectra of picolinamide, nicotinamide, and isonicotinamide. J Mol Struct. 2007;826(1):6–16.
Castro RAE, Ribeiro JD, Maria TM, Ramos Silva M, Yuste-Vivas C, Canotilho J, et al. Naproxen cocrystals with pyridinecarboxamide isomers. Cryst Growth Des. 2011;11(12):5396–404.
Wang L, Tan B, Zhang H, Deng Z. Pharmaceutical cocrystals of diflunisal with nicotinamide or isonicotinamide. Org Process Res Dev. 2013;17(11):1413–8.
Chow SF, Chen M, Shi L, Chow AH, Sun CC. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm Res. 2012;29(7):1854–65.
Ando S, Kikuchi J, Fujimura Y, Ida Y, Higashi K, Moribe K, Yamamoto K. Physicochemical characterization and structural evaluation of a specific 2: 1 cocrystal of naproxen–nicotinamide. J Pharm Sci. 2012;101(9):3214–21.
Parker FS. Applications of infrared, Raman, and resonance Raman spectroscopy in biochemistry. Springer Science & Business Media; 1983. pp. 568.
Lambert JB. Introduction to organic spectroscopy. Macmillan; 1987. pp. 454.
Glombitza BW, Oelkrug D, Schmidt PC. Surface acidity of solid pharmaceutical excipients. I: Determination of the surface acidity. Eur J Pharm Biopharm. 1994;40(5):289–93.
Jagia M, Daptardar R, Patel K, Bansal AK, Patel S. Role of Structure, Microenvironmental pH, and Speciation To Understand the Formation and Properties of Febuxostat Eutectics. Mol Pharm. 2019;16(11):4610–20.
Author information
Authors and Affiliations
Contributions
• Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: Moksh Jagia and Sarsvatkumar Patel
• Drafting the work or revising it critically for important intellectual content: Moksh Jagia and Dnyaneshwar P. Kale
• Final approval of the version to be published: Sarsvatkumar Patel and Arvind Kumar Bansal
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Moksh Jagia, Dnyaneshwar P. Kale, Sarsvatkumar Patel, and Arvind Kumar Bansal
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2595 kb)
Rights and permissions
About this article
Cite this article
Jagia, M., Kale, D.P., Bansal, A.K. et al. Novel Co-crystals and Eutectics of Febuxostat: Characterization, Mechanism of Formation, and Improved Dissolution. AAPS PharmSciTech 23, 43 (2022). https://doi.org/10.1208/s12249-021-02182-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02182-9