Abstract.
Amorphization is one of the most effective pharmaceutical approaches to enhance the dissolution and oral bioavailability of poorly water-soluble drugs. In recent years, amorphous formulations have been experiencing rapid development both in theoretical and practical application. Based on using different types of stabilizing agents, amorphous formulations can be mainly classified as polymer-based amorphous solid dispersion, coamorphous formulation, mesoporous silica-based amorphous formulation, etc. This paper summarizes recent advances in the dissolution and supersaturation of these amorphous formulations. Moreover, we also highlight the roles of stabilizing agents such as polymers, low molecular weight co-formers, and mesoporous silica. Maintaining supersaturation in solution is a key factor for the enhancement of dissolution profile and oral bioavailability, and thus, the strategies and challenges for maintaining supersaturation are also discussed. With an in-depth understanding of the inherent mechanisms of dissolution behaviors, the design of amorphous pharmaceutical formulations will become more scientific and reasonable, leading to vigorous development of commercial amorphous drug products.


Adapted from Ref. [50] with permission. Copyright © 2018 American Chemical Society

Adapted from Ref. [57] with permission. Copyright © 2016 American Chemical Society

Adapted from Ref. [62] with permission. Copyright © 2019 Elsevier

Adapted from Ref. [66] with permission. Copyright © 2015 American Chemical Society

Adapted from Ref. [86] with permission. Copyright © 2016 Elsevier

Adapted from Ref. [103] with permission. Copyright © 2020 American Chemical Society

Adapted from Ref. [104] with permission. Copyright © 2011 American Chemical Society

Adapted from Ref. [105] with permission. Copyright © 2020 Springer International Publishing

Adapted from Ref. [146] with permission. Copyright © 2018 American Chemical Society

Adapted from Ref. [150] with permission. Copyright © 2018 American Chemical Society
Similar content being viewed by others
REFERENCES
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17:486–95.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53.
Taylor LS, Zhang GG. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016;101:122–42.
Roni SA, Alejandro S. Electrohydrodynamic atomization and spray-drying for the production of pure drug nanocrystals and co-crystals. Adv Drug Deliv Rev. 2018;131:79–100.
Qiao N, Li M, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: an overview. Int J Pharm. 2011;419:1–11.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48:27–42.
Shi Q, Li F, Yeh S, Wang Y, Xin J. Physical stability of amorphous pharmaceutical solids: nucleation, crystal growth, phase separation and effects of the polymers. Int J Pharm. 2020;590:119925.
Trubitsyn G, Nguyen VN, Di Tommaso C, Borchard G, Gurny R, Moller M. Impact of covalently Nile Red and covalently rhodamine labeled fluorescent polymer micelles for the improved imaging of the respective drug delivery system. Eur J Pharm Biopharm. 2019;142:480–7.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2012;64:175–93.
Bazzo GC, Pezzini BR, Stulzer HK. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int J Pharm. 2020;588:119741.
Boyd BJ, Salim M, Clulow AJ, Ramirez G, Pham AC, Hawley A. The impact of digestion is essential to the understanding of milk as a drug delivery system for poorly water soluble drugs. J Control Release. 2018;292:13–7.
Han J, Wei Y, Lu Y, Wang R, Zhang J, Gao Y, Qian S. Co-amorphous systems for the delivery of poorly water-soluble drugs: recent advances and an update. Expert Opin Drug Deliv. 2020;17:1411–35.
Shi Q, Moinuddin SM, Cai T. Advances in coamorphous drug delivery systems. Acta Pharm Sin B. 2019;9:19–35.
Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J Pharm Sci. 2010;99:1254–64.
Descamps M, Dudognon E. Crystallization from the amorphous state: nucleation-growth decoupling, polymorphism interplay, and the role of interfaces. J Pharm Sci. 2014;103:2615–28.
Wyttenbach N, Kuentz M. Glass-forming ability of compounds in marketed amorphous drug products. Eur J Pharm Biopharm. 2017;112:204–8.
Moinuddin SM, Ruan S, Huang Y, Gao Q, Shi Q, Cai B, Cai T. Facile formation of co-amorphous atenolol and hydrochlorothiazide mixtures via cryogenic-milling: enhanced physical stability, dissolution and pharmacokinetic profile. Int J Pharm. 2017;532:393–400.
Qian S, Heng W, Wei Y, Zhang J, Gao Y. Coamorphous lurasidone hydrochloride–saccharin with charge-assisted hydrogen bonding interaction shows improved physical stability and enhanced dissolution with pH-independent solubility behavior. Cryst Growth Des. 2015;15:2920–8.
McCarthy CA, Ahern RJ, Dontireddy R, Ryan KB, Crean AM. Mesoporous silica formulation strategies for drug dissolution enhancement: a review. Expert Opin Drug Deliv. 2016;13:93–108.
Maleki A, Kettiger H, Schoubben A, Rosenholm JM, Ambrogi V, Hamidi M. Mesoporous silica materials: from physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J Control Release. 2017;262:329–47.
Huang C, Powell CT, Sun Y, Cai T, Yu L. Effect of low-concentration polymers on crystal growth in molecular glasses: a controlling role for polymer segmental mobility relative to host dynamics. J Phys Chem B. 2017;121:1963–71.
Powell CT, Cai T, Hasebe M, Gunn EM, Gao P, Zhang G, Gong Y, Yu L. Low-concentration polymers inhibit and accelerate crystal growth in organic glasses in correlation with segmental mobility. J Phys Chem B. 2013;117:10334–41.
Fung MH, DeVault M, Kuwata KT, Suryanarayanan R. Drug-excipient interactions: effect on molecular mobility and physical stability of ketoconazole-organic acid coamorphous systems. Mol Pharm. 2018;15:1052–61.
Mistry P, Suryanarayanan R. Strength of drug–polymer interactions: implications for crystallization in dispersions. Cryst Growth Des. 2016;16:5141–9.
Zhang C, Sha Y, Zhang Y, Cai T, Li L, Zhou D, Wang X, Xue G. Nanostructures and dynamics of isochorically confined amorphous drug mediated by cooling rate, interfacial, and intermolecular interactions. J Phys Chem B. 2017;121:10704–16.
He Y, Zhang W, Guo T, Zhang G, Qin W, Zhang L, Wang C, Zhu W, Yang M, Hu X, Singh V, Wu L, Gref R, Zhang J. Drug nanoclusters formed in confined nano-cages of CD-MOF: dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm Sin B. 2019;9:97–106.
Huang Y, Dai WG. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4:18–25.
Dahan A, Beig A, Lindley D, Miller JM. The solubility-permeability interplay and oral drug formulation design: two heads are better than one. Adv Drug Deliv Rev. 2016;101:99–107.
Beig A, Miller JM, Lindley D, Carr RA, Zocharski P, Agbaria R, Dahan A. Head-to-head comparison of different solubility-enabling formulations of etoposide and their consequent solubility-permeability interplay. J Pharm Sci. 2015;104:2941–7.
Miller JM, Dahan A. Predicting the solubility-permeability interplay when using cyclodextrins in solubility-enabling formulations: model validation. Int J Pharm. 2012;430:388–91.
Dahan A, Miller JM. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14:244–51.
Paus R, Ji Y, Vahle L, Sadowski G. Predicting the solubility advantage of amorphous pharmaceuticals: a novel thermodynamic approach. Mol Pharm. 2015;12:2823–33.
Enright EF, Joyce SA, Gahan CGM, Taylor LS. Impact of phospholipid digests and bile acid pool variations on the crystallization of atazanavir from supersaturated solutions. Eur J Pharm Biopharm. 2020;153:68–83.
Baghel S, Cathcart H, O’Reilly NJ. Theoretical and experimental investigation of drug-polymer interaction and miscibility and its impact on drug supersaturation in aqueous medium. Eur J Pharm Biopharm. 2016;107:16–31.
Van Eerdenbrugh B, Raina S, Hsieh YL, Augustijns P, Taylor LS. Classification of the crystallization behavior of amorphous active pharmaceutical ingredients in aqueous environments. Pharm Res. 2014;31:969–82.
Baird JA, Van Eerdenbrugh B, Taylor LS. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J Pharm Sci. 2010;99:3787–806.
Mehta M, Kothari K, Ragoonanan V, Suryanarayanan R. Effect of water on molecular mobility and physical stability of amorphous pharmaceuticals. Mol Pharm. 2016;13:1339–46.
Li H, Zhang M, Xiong L, Feng W, Williams RO. Bioavailability improvement of carbamazepine via oral administration of modified-release amorphous solid dispersions in rats. Pharmaceutics. 2020;12:1203.
Chen Y, Wang S, Wang S, Liu C, Su C, Hageman M, Hussain M, Haskell R, Stefanski K, Qian F. Initial drug dissolution from amorphous solid dispersions controlled by polymer dissolution and drug-polymer interaction. Pharm Res. 2016;33:2445–58.
Alonzo DE, Gao Y, Zhou D, Mo H, Zhang GG, Taylor LS. Dissolution and precipitation behavior of amorphous solid dispersions. J Pharm Sci. 2011;100:3316–31.
Saboo S, Moseson DE, Kestur US, Taylor LS. Patterns of drug release as a function of drug loading from amorphous solid dispersions: a comparison of five different polymers. Eur J Pharm Sci. 2020;155:105514.
Konno H, Handa T, Alonzo DE, Taylor LS. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70:493–9.
Jackson MJ, Kestur US, Hussain MA, Taylor LS. Dissolution of danazol amorphous solid dispersions: supersaturation and phase behavior as a function of drug loading and polymer type. Mol Pharm. 2016;13:223–31.
Bevernage J, Forier T, Brouwers J, Tack J, Annaert P, Augustijns P. Excipient-mediated supersaturation stabilization in human intestinal fluids. Mol Pharm. 2011;8:564–70.
Xie S, Poornachary SK, Chow PS, Tan RBH. Direct precipitation of micron-size salbutamol sulfate: new insights into the action of surfactants and polymeric additives. Cryst Growth Des. 2010;10:3363–71.
Yani Y, Chow PS, Tan RBH. Molecular simulation study of the effect of various additives on salbutamol sulfate crystal habit. Mol Pharm. 2011;8:1910–8.
Que C, Gao Y, Raina SA, Zhang GG, Taylor LS. Paclitaxel crystal seeds with different intrinsic properties and their impact on dissolution of paclitaxel-HPMCAS amorphous solid dispersions. Cryst Growth Des. 2018;18:1548–59.
Chen Y, Liu C, Chen Z, Su C, Hageman M, Hussain M, Haskell R, Stefanski K, Qian F. Drug-polymer-water interaction and its implication for the dissolution performance of amorphous solid dispersions. Mol Pharm. 2015;12:576–89.
Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Impact of polymers on crystal growth rate of structurally diverse compounds from aqueous solution. Mol Pharm. 2013;10:2381–93.
Pui Y, Chen Y, Chen H, Wang S, Liu C, Tonnis W, Chen L, Serno P, Bracht S, Qian F. Maintaining supersaturation of nimodipine by PVP with or without the presence of sodium lauryl sulfate and sodium taurocholate. Mol Pharm. 2018;15:2754–63.
Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Understanding polymer properties important for crystal growth inhibition—impact of chemically diverse polymers on solution crystal growth of ritonavir. Cryst Growth Des. 2012;12:3133–43.
Wang S, Liu C, Chen Y, Zhu A, Qian F. Aggregation of hydroxypropyl methylcellulose acetate succinate under its dissolving pH and the impact on drug supersaturation. Mol Pharm. 2018;15:4643–53.
Schram CJ, Beaudoin SP, Taylor LS. Impact of polymer conformation on the crystal growth inhibition of a poorly water-soluble drug in aqueous solution. Langmuir. 2015;31:171–9.
Ueda K, Hate SS, Taylor LS. Impact of hypromellose acetate succinate grade on drug amorphous solubility and in vitro membrane transport. J Pharm Sci. 2020;109:2464–73.
Sugihara H, Taylor LS. Evaluation of pazopanib phase behavior following pH-induced supersaturation. Mol Pharm. 2018;15:1690–9.
Amponsah-Efah KK, Mistry P, Eisenhart R, Suryanarayanan R. The influence of the strength of drug–polymer interactions on the dissolution of amorphous solid dispersions. Mol Pharm. 2021;18:174–86.
Chen Y, Wang S, Wang S, Liu C, Su C, Hageman M, Hussain M, Haskell R, Stefanski K, Qian F. Sodium lauryl sulfate competitively interacts with HPMC-AS and consequently reduces oral bioavailability of posaconazole/HPMC-AS amorphous solid dispersion. Mol Pharm. 2016;13:2787–95.
Liu C, Chen Z, Chen Y, Lu J, Li Y, Wang S, Wu G, Qian F. Improving oral bioavailability of sorafenib by optimizing the “spring” and “parachute” based on molecular interaction mechanisms. Mol Pharm. 2016;13:599–608.
Ilevbare GA, Taylor LS. Liquid–liquid phase separation in highly supersaturated aqueous solutions of poorly water-soluble drugs: Implications for solubility enhancing formulations. Cryst Growth Des. 2013;13:1497–509.
Wallace AF, Hedges LO, Fernandez-Martinez A, Raiteri P, Gale JD, Waychunas GA, Whitelam S, Banfield JF, De Yoreo JJ. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions. Science. 2013;341:885–9.
Lafferrère L, Hoff C, Veesler S. Study of liquid–liquid demixing from drug solution. J Cryst Growth. 2004;269:550–7.
Saboo S, Mugheirbi NA, Zemlyanov DY, Kestur US, Taylor LS. Congruent release of drug and polymer: a “sweet spot” in the dissolution of amorphous solid dispersions. J Control Release. 2019;298:68–82.
Wilson V, Lou X, Osterling DJ, Stolarik DF, Jenkins G, Gao W, Zhang GG, Taylor LS. Relationship between amorphous solid dispersion in vivo absorption and in vitro dissolution: phase behavior during dissolution, speciation, and membrane mass transport. J Control Release. 2018;292:172–82.
Mosquera-Giraldo LI, Taylor LS. Glass-liquid phase separation in highly supersaturated aqueous solutions of telaprevir. Mol Pharm. 2015;12:496–503.
Mosquera-Giraldo LI, Li N, Wilson VR, Nichols BLB, Edgar KJ, Taylor LS. Influence of polymer and drug loading on the release profile and membrane transport of telaprevir. Mol Pharm. 2018;15:1700–13.
Almeida e Sousa L, Reutzel-Edens SM, Stephenson GA, Taylor LS. Assessment of the amorphous “solubility” of a group of diverse drugs using new experimental and theoretical approaches. Mol Pharm. 2015;12:484–95.
Raina SA, Zhang GG, Alonzo DE, Wu J, Zhu D, Catron ND, Gao Y, Taylor LS. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J Pharm Sci. 2014;103:2736–48.
Taboada P, Attwood D, Ruso JM, García M, Mosquera V. Static and dynamic light scattering study on the association of some antidepressants in aqueous electrolyte solutions. Phys Chem Chem Phys. 2000;2:5175–9.
Raina SA, Alonzo DE, Zhang GG, Gao Y, Taylor LS. Using environment-sensitive fluorescent probes to characterize liquid-liquid phase separation in supersaturated solutions of poorly water soluble compounds. Pharm Res. 2015;32:3660–73.
Tres F, Hall SD, Mohutsky MA, Taylor LS. Monitoring the phase behavior of supersaturated solutions of poorly water-soluble drugs using fluorescence techniques. J Pharm Sci. 2018;107:94–102.
Tres F, Posada MM, Hall SD, Mohutsky MA, Taylor LS. Mechanistic understanding of the phase behavior of supersaturated solutions of poorly water-soluble drugs. Int J Pharm. 2018;543:29–37.
Sugano K, Kato T, Suzuki K, Keiko K, Sujaku T, Mano T. High throughput solubility measurement with automated polarized light microscopy analysis. J Pharm Sci. 2006;95:2115–22.
Qi S, Roser S, Edler KJ, Pigliacelli C, Rogerson M, Weuts I, Van Dycke F, Stokbroekx S. Insights into the role of polymer-surfactant complexes in drug solubilisation/stabilisation during drug release from solid dispersions. Pharm Res. 2013;30:290–302.
Gao X, Huang Y, Makhov AM, Epperly M, Lu J, Grab S, Zhang P, Rohan L, Xie X, Wipf P. Nano-assembly of surfactants with interfacial drug-interactive motifs as tailor-designed drug carriers. Mol Pharm. 2013;10:187–98.
Giannetti AM, Koch BD, Browner MF. Surface plasmon resonance based assay for the detection and characterization of promiscuous inhibitors. J Med Chem. 2008;51:574–80.
Laitinen R, Lobmann K, Grohganz H, Priemel P, Strachan CJ, Rades T. Supersaturating drug delivery systems: the potential of co-amorphous drug formulations. Int J Pharm. 2017;532:1–12.
Ueda K, Yamamoto N, Higashi K, Moribe K. Molecular mobility suppression of ibuprofen-rich amorphous nanodroplets by HPMC revealed by NMR relaxometry and its significance with respect to crystallization inhibition. Mol Pharm. 2019;16:4968–77.
Raina SA, Eerdenbrugh BV, Alonzo DE, Mo H, Zhang GG, Gao Y, Taylor LS. Trends in the precipitation and crystallization behavior of supersaturated aqueous solutions of poorly water-soluble drugs assessed using synchrotron radiation. J Pharm Sci. 2015;104:1981–92.
Alonzo DE, Raina S, Zhou D, Gao Y, Zhang GG, Taylor LS. Characterizing the impact of hydroxypropylmethyl cellulose on the growth and nucleation kinetics of felodipine from supersaturated solutions. Cryst Growth Des. 2012;12:1538–47.
Ilevbare GA, Liu H, Pereira J, Edgar KJ, Taylor LS. Influence of additives on the properties of nanodroplets formed in highly supersaturated aqueous solutions of ritonavir. Mol Pharm. 2013;10:3392–403.
Trasi NS, Taylor LS. Dissolution performance of binary amorphous drug combinations–Impact of a second drug on the maximum achievable supersaturation. Int J Pharm. 2015;496:282–90.
Van Zee NJ, Hillmyer MA, Lodge TP. Role of polymer excipients in the kinetic stabilization of drug-rich nanoparticles. ACS Appl BioMater. 2020;3:7243–54.
Ueda K, Taylor LS. Partitioning of surfactant into drug-rich nanodroplets and its impact on drug thermodynamic activity and droplet size. J Control Release. 2021;330:229–43.
Li N, Taylor LS. Tailoring supersaturation from amorphous solid dispersions. J Control Release. 2018;279:114–25.
Arca HC, Mosquera-Giraldo LI, Dahal D, Taylor LS, Edgar KJ. Multidrug, anti-HIV amorphous solid dispersions: nature and mechanisms of impacts of drugs on each other’s solution concentrations. Mol Pharm. 2017;14:3617–27.
Alhalaweh A, Bergstrom CAS, Taylor LS. Compromised in vitro dissolution and membrane transport of multidrug amorphous formulations. J Control Release. 2016;229:172–82.
El Sayed M, Alhalaweh A, Bergstrom CAS. Insights into dissolution and solution chemistry of multidrug formulations of antihypertensive drugs. Mol Pharm. 2020;17:4018–28.
Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Maintaining supersaturation in aqueous drug solutions: Impact of different polymers on induction times. Cryst Growth Des. 2013;13:740–51.
Curatolo W, Nightingale JA, Herbig SM. Utility of hydroxypropylmethylcellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI milieu. Pharm Res. 2009;26:1419–31.
Vasconcelos T, Marques S, das Neves J, Sarmento B. Amorphous solid dispersions: rational selection of a manufacturing process. Adv Drug Deliv Rev. 2016;100:85–101.
Newman A, Reutzel-Edens SM, Zografi G. Coamorphous active pharmaceutical ingredient-small molecule mixtures: considerations in the choice of coformers for enhancing dissolution and oral bioavailability. J Pharm Sci. 2018;107:5–17.
Wang R, Han J, Jiang A, Huang R, Fu T, Wang L, Zheng Q, Li W, Li J. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int J Pharm. 2019;561:9–18.
Korhonen O, Pajula K, Laitinen R. Rational excipient selection for co-amorphous formulations. Expert Opin Drug Deliv. 2017;14:551–69.
Mkc M, Suresh K, Kumar BM, Bhavani KD, Nangia A. Curcumin-artemisinin coamorphous solid: xenograft model preclinical study. Pharmaceutics. 2018;10:7.
Yu D, Kan Z, Shan F, Zang J, Zhou J. Triple strategies to improve oral bioavailability by fabricating coamorphous forms of ursolic acid with piperine: enhancing water-solubility, permeability, and inhibiting cytochrome P450 isozymes. Mol Pharm. 2020;17:4443–62.
Alleso M, Chieng N, Rehder S, Rantanen J, Rades T, Aaltonen J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: amorphous naproxen-cimetidine mixtures prepared by mechanical activation. J Control Release. 2009;136:45–53.
Dengale SJ, Ranjan OP, Hussen SS, Krishna BS, Musmade PB, Gautham Shenoy G, Bhat K. Preparation and characterization of co-amorphous Ritonavir-Indomethacin systems by solvent evaporation technique: improved dissolution behavior and physical stability without evidence of intermolecular interactions. Eur J Pharm Sci. 2014;62:57–64.
Gniado K, Lobmann K, Rades T, Erxleben A. The influence of co-formers on the dissolution rates of co-amorphous sulfamerazine/excipient systems. Int J Pharm. 2016;504:20–6.
Haneef J, Chadha R. Drug-drug multicomponent solid forms: cocrystal, coamorphous and eutectic of three poorly soluble antihypertensive drugs using mechanochemical approach. AAPS PharmSciTech. 2017;18:2279–90.
Kasten G, Nouri K, Grohganz H, Rades T, Löbmann K. Performance comparison between crystalline and co-amorphous salts of indomethacin-lysine. Int J Pharm. 2017;533:138–44.
Jensen KT, Blaabjerg LI, Lenz E, Bohr A, Grohganz H, Kleinebudde P, Rades T, Löbmann K. Preparation and characterization of spray-dried co-amorphous drug-amino acid salts. J Pharm Pharmacol. 2016;68:615–24.
Heng W, Wei Y, Xue Y, Cheng H, Zhang L, Zhang J, Gao Y, Qian S. Gel formation induced slow dissolution of amorphous indomethacin. Pharm Res. 2019;36:159.
Heng W, Su M, Cheng H, Shen P, Liang S, Zhang L, Wei Y, Gao Y, Zhang J, Qian S. Incorporation of complexation into a coamorphous system dramatically enhances dissolution and eliminates gelation of amorphous lurasidone hydrochloride. Mol Pharm. 2020;17:84–97.
Lobmann K, Laitinen R, Grohganz H, Gordon KC, Strachan C, Rades T. Coamorphous drug systems: enhanced physical stability and dissolution rate of indomethacin and naproxen. Mol Pharm. 2011;8:1919–28.
Moinuddin SM, Shi Q, Tao J, Guo M, Zhang J, Xue Q, Ruan S, Cai T. Enhanced physical stability and synchronized release of febuxostat and indomethacin in coamorphous solids. AAPS PharmSciTech. 2020;21:41.
Wang S, Heng W, Wang X, He X, Zhang Z, Wei Y, Zhang J, Gao Y, Qian S. Coamorphization combined with complexation enhances dissolution of lurasidone hydrochloride and puerarin with synchronized release. Int J Pharm. 2020;588:119793.
Skieneh JM, Sathisaran I, Dalvi SV, Rohani S. Co-amorphous form of curcumin–folic acid dihydrate with increased dissolution rate. Cryst Growth Des. 2017;17:6273–80.
Shayanfar A, Ghavimi H, Hamishekar H, Jouyban A. Coamorphous atorvastatin calcium to improve its physicochemical and pharmacokinetic properties. J Pharm Pharm Sci. 2013;16:577–87.
Paluch KJ, Mccabe T, Müller-Bunz H, Corrigan OI, Healy AM, Tajber L. Formation and physicochemical properties of crystalline and amorphous salts with different stoichiometries formed between ciprofloxacin and succinic acid. Mol Pharm. 2013;10:2654–3640.
Fung MH, Berzins K, Suryanarayanan R. Physical stability and dissolution behavior of ketoconazole-organic acid coamorphous systems. Mol Pharm. 2018;15:1862–9.
Li Z, Zhang Y, Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv. 2019;16:219–37.
Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed Engl. 2007;46:7548–58.
Xu W, Riikonen J, Lehto VP. Mesoporous systems for poorly soluble drugs. Int J Pharm. 2013;453:181–97.
Sun W, Aburub A, Sun CC. Particle engineering for enabling a formulation platform suitable for manufacturing low dose tablets by direct compression. J Pharm Sci. 2017;106:1772–7.
Sun W, Aburub A, Sun CC. A mesoporous silica based platform to enable tablet formulations of low dose drugs by direct compression. Int J Pharm. 2018;539:184–9.
Schenck L, Erdemir D, Gorka LS, Merritt JM, Marziano I, Ho R, Lee M, Bullard J, Boukerche M, Ferguson S, Florence AJ, Khan SA, Sun CC. Recent advances in co-processed APIs and proposals for enabling commercialization of these transformative technologies. Mol Pharm. 2020;17:2232–44.
Bukara K, Schueller L, Rosier J, Martens MA, Daems T, Verheyden L, Eelen S, Van Speybroeck M, Libanati C, Martens JA, Van Den Mooter G, Frerart F, Jolling K, De Gieter M, Bugarski B, Kiekens F. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: proof of concept in man. Eur J Pharm Biopharm. 2016;108:220–5.
Izquierdo-Barba I, Martinez A, Doadrio AL, Perez-Pariente J, Vallet-Regi M. Release evaluation of drugs from ordered three-dimensional silica structures. Eur J Pharm Sci. 2005;26:365–73.
Kumar D, Sailaja Chirravuri SV, Shastri NR. Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water soluble drugs: a case study with aceclofenac. Int J Pharm. 2014;461:459–68.
Mellaerts R, Aerts CA, Van Humbeeck J, Augustijns P, Van den Mooter G, Martens JA. Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials. Chem Commun. 2007;13:1375–7.
Shen SC, Ng WK, Chia L, Hu J, Tan RB. Physical state and dissolution of ibuprofen formulated by co-spray drying with mesoporous silica: effect of pore and particle size. Int J Pharm. 2011;410:188–95.
Andersson J, Rosenholm J, Areva S, Lindén M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro- and mesoporous silica matrice. Chem Mater. 2004;16:4160–7.
Maleki A, Hamidi M. Dissolution enhancement of a model poorly water-soluble drug, atorvastatin, with ordered mesoporous silica: comparison of MSF with SBA-15 as drug carriers. Expert Opin Drug Deliv. 2016;13:171–81.
Hu Y, Zhi Z, Zhao Q, Wu C, Zhao P, Jiang H, Jiang T, Wang S. 3D cubic mesoporous silica microsphere as a carrier for poorly soluble drug carvedilol. Microporous Mesoporous Mater. 2012;147:94–101.
Rosenholm JM, Linden M. Towards establishing structure-activity relationships for mesoporous silica in drug delivery applications. J Control Release. 2008;128:157–64.
Eren ZS, Tunçer S, Gezer G, Yildirim LT, Banerjee S, Yilmaz A. Improved solubility of celecoxib by inclusion in SBA-15 mesoporous silica: drug loading in different solvents and release. Microporous Mesoporous Mater. 2016;235:211–23.
Jambhrunkar S, Qu Z, Popat A, Karmakar S, Xu C, Yu C. Modulating in vitro release and solubility of griseofulvin using functionalized mesoporous silica nanoparticles. J Colloid Interface Sci. 2014;434:218–25.
Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev. 2012;41:3679–98.
Waters LJ, Hussain T, Parkes G, Hanrahan JP, Tobin JM. Inclusion of fenofibrate in a series of mesoporous silicas using microwave irradiation. Eur J Pharm Biopharm. 2013;85:936–41.
Ahern RJ, Crean AM, Ryan KB. The influence of supercritical carbon dioxide (SC-CO2) processing conditions on drug loading and physicochemical properties. Int J Pharm. 2012;439:92–9.
Shen SC, Ng WK, Chia L, Dong YC, Tan RB. Stabilized amorphous state of ibuprofen by co-spray drying with mesoporous SBA-15 to enhance dissolution properties. J Pharm Sci. 2010;99:1997–2007.
Koch N, Jennotte O, Grignard B, Lechanteur A, Evrard B. Impregnation of mesoporous silica with poor aqueous soluble molecule using pressurized carbon dioxide: is the solubility in the supercritical and subcritical phase a critical parameter? Eur J Pharm Sci. 2020;150:105332.
Ukmar T, Maver U, Planinsek O, Kaucic V, Gaberscek M, Godec A. Understanding controlled drug release from mesoporous silicates: theory and experiment. J Control Release. 2011;155:409–17.
Kinnari P, Mäkilä E, Heikkilä T, Salonen J, Hirvonen J, Santos HA. Comparison of mesoporous silicon and non-ordered mesoporous silica materials as drug carriers for itraconazole. Int J Pharm. 2011;414:148–56.
Xue JM, Shi M. PLGA/mesoporous silica hybrid structure for controlled drug release. J Control Release. 2004;98:209–17.
Qian KK, Bogner RH. Application of mesoporous silicon dioxide and silicate in oral amorphous drug delivery systems. J Pharm Sci. 2012;101:444–63.
Rengarajan GT, Enke D, Steinhart M, Beiner M. Stabilization of the amorphous state of pharmaceuticals in nanopores. J Mater Chem. 2008;18:2537–9.
Bahl D, Bogner RH. Amorphization of indomethacin by co-grinding with neusilin US2: amorphization kinetics, physical stability and mechanism. Pharm Res. 2006;23:2317–25.
Mužík J, Lizoňová D, Zadražil A, Štěpánek F. Drug amorphisation by fluid bed hot-melt impregnation of mesoporous silica carriers. Chem Eng J. 2020;392:123754.
Hempel NJ, Brede K, Olesen NE, Genina N, Knopp MM, Lobmann K. A fast and reliable DSC-based method to determine the monomolecular loading capacity of drugs with good glass-forming ability in mesoporous silica. Int J Pharm. 2018;544:153–7.
Ahern RJ, Hanrahan JP, Tobin JM, Ryan KB, Crean AM. Comparison of fenofibrate-mesoporous silica drug-loading processes for enhanced drug delivery. Eur J Pharm Sci. 2013;50:400–9.
Ren X, Cheng S, Liang Y, Yu X, Sheng J, Wan Y, Li Y, Wan J, Luo Z, Yang X. Mesoporous silica nanospheres as nanocarriers for poorly soluble drug itraconazole with high loading capacity and enhanced bioavailability. Microporous Mesoporous Mater. 2020;305:110389.
Van SM, Barillaro V, Thi TD, Mellaerts R, Martens J, Van HJ, Vermant J, Annaert P, Van den Mooter G, Augustijns P. Ordered mesoporous silica material SBA-15: a broad-spectrum formulation platform for poorly soluble drugs. J Pharm Sci. 2009;98:2648–58.
Limnell T, Santos HA, Makila E, Heikkila T, Salonen J, Murzin DY, Kumar N, Laaksonen T, Peltonen L, Hirvonen J. Drug delivery formulations of ordered and nonordered mesoporous silica: comparison of three drug loading methods. J Pharm Sci. 2011;100:3294–306.
Dening TJ, Zemlyanov D, Taylor LS. Application of an adsorption isotherm to explain incomplete drug release from ordered mesoporous silica materials under supersaturating conditions. J Control Release. 2019;307:186–99.
McCarthy CA, Ahern RJ, Devine KJ, Crean AM. Role of drug adsorption onto the silica surface in drug release from mesoporous silica systems. Mol Pharm. 2018;15:141–9.
McCarthy CA, Zemlyanov DY, Crean AM, Taylor LS. Comparison of drug release and adsorption under supersaturating conditions for ordered mesoporous silica with indomethacin or indomethacin methyl ester. Mol Pharm. 2020;17:3062–74.
Hate SS, Reutzel-Edens SM, Taylor LS. Influence of drug-silica electrostatic interactions on drug release from mesoporous silica-based oral delivery systems. Mol Pharm. 2020;17:3435–46.
Mellaerts R, Mols R, Kayaert P, Annaert P, Van Humbeeck J, Van den Mooter G, Martens JA, Augustijns P. Ordered mesoporous silica induces pH-independent supersaturation of the basic low solubility compound itraconazole resulting in enhanced transepithelial transport. Int J Pharm. 2008;357:169–79.
Dening TJ, Taylor LS. Supersaturation potential of ordered mesoporous silica delivery systems. Part 1: dissolution performance and drug membrane transport rates. Mol Pharm. 2018;15:3489–501.
Hate SS, Reutzel-Edens SM, Taylor LS. Interplay of adsorption, supersaturation and the presence of an absorptive sink on drug release from mesoporous silica-based formulations. Pharm Res. 2020;37:163.
Wu Q, Feng D, Huang Z, Chen M, Yang D, Pan X, Lu C, Quan G, Wu C. Supersaturable organic-inorganic hybrid matrix based on well-ordered mesoporous silica to improve the bioavailability of water insoluble drugs. Drug Deliv. 2020;27:1292–300.
Price DJ, Nair A, Becker-Baldus J, Glaubitz C, Kuentz M, Dressman J, Saal C. Incorporation of HPMCAS during loading of glibenclamide onto mesoporous silica improves dissolution and inhibits precipitation. Eur J Pharm Sci. 2020;141:105113.
Yang M, Chen T, Wang L, Chen L, Li J, Di L. High dispersed phyto-phospholipid complex/TPGS 1000 with mesoporous silica to enhance oral bioavailability of tanshinol. Colloid Surf B. 2018;170:187–93.
Li Z, Van Zee NJ, Bates FS, Lodge TP. Polymer nanogels as reservoirs to inhibit hydrophobic drug crystallization. ACS Nano. 2019;13:1232–43.
Li Z, Johnson LM, Ricarte RG, Yao LJ, Hillmyer MA, Bates FS, Lodge TP. Enhanced performance of blended polymer excipients in delivering a hydrophobic drug through the synergistic action of micelles and HPMCAS. Langmuir. 2017;33:2837–48.
Li Z, Lenk TI, Yao LJ, Bates FS, Lodge TP. Maintaining hydrophobic drug supersaturation in a micelle corona reservoir. Macromolecules. 2018;51:540–51.
Huang R, Han J, Wang R, Zhao X, Qiao H, Chen L, Li W, Di L, Zhang W, Li J. Surfactant-free solid dispersion of BCS class IV drug in an amorphous chitosan oligosaccharide matrix for concomitant dissolution in vitro—permeability increase. Eur J Pharm Sci. 2019;130:147–55.
Sahbaz Y, Williams HD, Nguyen T, Saunders J, Ford L, Charman SA, Scammells PJ, Porter CJH. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol Pharm. 2015;12:1980–91.
Stocker MW, Healy AM, Ferguson A. Spray encapsulation as a formulation strategy for drug-based room temperature ionic liquids: exploiting drug−polymer immiscibility to enable processing for solid dosage forms. Mol Pharm. 2020;17:3412–24.
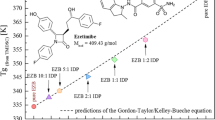
Elkhabaz A, Sarkar S, Dinh JK, Simpson GJ, Taylor LS. Variation in supersaturation and phase behavior of ezetimibe amorphous solid dispersions upon dissolution in different biorelevant media. Mol Pharm. 2018;15:193–206.
Chen J, Mosquera-Giraldo LI, Ormes JD, Higgins JD, Taylor LS. Bile salts as crystallization inhibitors of supersaturated solutions of poorly water-soluble compounds. Cryst Growth Des. 2015;15:2593–7.
Lu J, Ormes JD, Lowinger M, Mann AKP, Xu W, Litster JD, Taylor LS. Maintaining supersaturation of active pharmaceutical ingredient solutions with biologically relevant bile salts. Cryst Growth Des. 2017;17:2782–91.
Lu J, Ormes JD, Lowinger M, Mann AKP, Xu W, Patel S, Litster JD, Taylor LS. Impact of bile salts on solution crystal growth rate and residual supersaturation of an active pharmaceutical ingredient. Cryst Growth Des. 2017;17:3528–37.
Liu C, Liu Z, Chen Y, Chen Z, Chen H, Pui Y, Qian F. Oral bioavailability enhancement of beta-lapachone, a poorly soluble fast crystallizer, by cocrystal, amorphous solid dispersion, and crystalline solid dispersion. Eur J Pharm Biopharm. 2018;124:73–81.
ACKNOWLEDGEMENTS
The authors are grateful for the financial support of this work by the National Natural Science Foundation of China (No.81803452), the Natural Science General Project of Jiangsu Provincial Department of Education (No.18KJB360015) and National Subject Cultivation Project of Jiangsu Vocational College of Medicine (No.20204304). Q.S., J.X.(Junbo Xin) and H.C are also grateful for the financial support of this work by College Student Innovation and Entrepreneurship Training Program of Jiangsu Province (No. 202012682008Y).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, Q., Li, F., Yeh, S. et al. Recent Advances in Enhancement of Dissolution and Supersaturation of Poorly Water-Soluble Drug in Amorphous Pharmaceutical Solids: A Review. AAPS PharmSciTech 23, 16 (2022). https://doi.org/10.1208/s12249-021-02137-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02137-0