Abstract
Polymeric amorphous solid dispersion (ASD) is a popular approach for enhancing the solubility of poorly water-soluble drugs. However, achieving both physical stability and dissolution performance in an ASD prepared with a single polymer can be challenging. Therefore, a secondary excipient can be added. In this paper, we review three classes of additives that can be added internally to ASDs: (i) a second polymer, to form a ternary drug-polymer–polymer ASD, (ii) counterions, to facilitate in situ salt formation, and (iii) surfactants. In an ASD prepared with a combination of polymers, each polymer exerts a unique function, such as a stabilizer in the solid state and a crystallization inhibitor during dissolution. In situ salt formation in ASD usually leads to substantial increases in the glass transition temperature, contributing to improved physical stability. Surfactants can enhance the wettability of ASD particles, thereby promoting rapid drug release. However, their potential adverse effects on physical stability and dissolution, resulting from enhanced molecular mobility and competitive molecular interaction with the polymer, respectively, warrant careful consideration. Finally, we discuss the impact of magnesium stearate and inorganic salts, excipients added externally upon downstream processing, on the solid-state stability as well as the dissolution of ASD tablets.
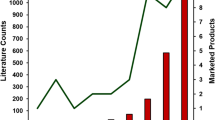
Graphical Abstract








Similar content being viewed by others
References
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53.
Baghel S, Cathcart H, O’Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105:2527–44.
Taylor LS, Zhang GGZ. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016;101:122–42.
Saboo S, Mugheirbi NA, Zemlyanov DY, Kestur US, Taylor LS. Congruent release of drug and polymer: a “sweet spot” in the dissolution of amorphous solid dispersions. J Control Release. 2019;298:68–82.
Indulkar AS, Gao Y, Raina SA, Zhang GGZ, Taylor LS. Exploiting the phenomenon of liquid–liquid phase separation for enhanced and sustained membrane transport of a poorly water-soluble drug. Mol Pharm. 2016;13:2059–69.
Chen Y, Liu C, Chen Z, Su C, Hageman M, Hussain M, et al. Drug–polymer–water interaction and its implication for the dissolution performance of amorphous solid dispersions. Mol Pharm. 2015;12:576–89.
Kwon, Giri, Song, Bae, Lee, Kim. Spray-dried amorphous solid dispersions of atorvastatin calcium for improved supersaturation and oral bioavailability. Pharmaceutics. 2019;11:461.
Indulkar AS, Lou X, Zhang GGZ, Taylor LS. Insights into the dissolution mechanism of ritonavir–copovidone amorphous solid dispersions: importance of congruent release for enhanced performance. Mol Pharm. 2019;16:1327–39.
Saboo S, Moseson DE, Kestur US, Taylor LS. Patterns of drug release as a function of drug loading from amorphous solid dispersions: a comparison of five different polymers. Eur J Pharm Sci. 2020;155: 105514.
Sahoo A, Suryanarayanan R, Siegel RA. Stabilization of amorphous drugs by polymers: the role of overlap concentration (C *). Mol Pharm. 2020;17:4401–6.
Mistry P, Mohapatra S, Gopinath T, Vogt FG, Suryanarayanan R. Role of the strength of drug–polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol Pharm. 2015;12:3339–50.
Kothari K, Ragoonanan V, Suryanarayanan R. The role of drug–polymer hydrogen bonding interactions on the molecular mobility and physical stability of nifedipine solid dispersions. Mol Pharm. 2015;12:162–70.
Sarabu S, Kallakunta VR, Bandari S, Batra A, Bi V, Durig T, et al. Hypromellose acetate succinate based amorphous solid dispersions via hot melt extrusion: effect of drug physicochemical properties. Carbohydr Polym. 2020;233: 115828.
Hiew TN, Zemlyanov DY, Taylor LS. Balancing solid-state stability and dissolution performance of lumefantrine amorphous solid dispersions: the role of polymer choice and drug–polymer interactions. Mol Pharm. 2022;19:392–413.
Liu L, Chen L, Müllers W, Serno P, Qian F. Water-resistant drug–polymer interaction contributes to the formation of nano-species during the dissolution of felodipine amorphous solid dispersions. Mol Pharm. 2022;19:2888–99.
Chen Y, Pui Y, Chen H, Wang S, Serno P, Tonnis W, et al. Polymer-mediated drug supersaturation controlled by drug–polymer interactions persisting in an aqueous environment. Mol Pharm. 2019;16:205–13.
Amponsah-Efah KK, Mistry P, Eisenhart R, Suryanarayanan R. The influence of the strength of drug–polymer interactions on the dissolution of amorphous solid dispersions. Mol Pharm. 2021;18:174–86.
Saboo S, Kestur US, Flaherty DP, Taylor LS. Congruent release of drug and polymer from amorphous solid dispersions: insights into the role of drug-polymer hydrogen bonding, surface crystallization, and glass transition. Mol Pharm. 2020;17:1261–75.
Que C, Deac A, Zemlyanov DY, Qi Q, Indulkar AS, Gao Y, et al. Impact of drug–polymer intermolecular interactions on dissolution performance of copovidone-based amorphous solid dispersions. Mol Pharm. 2021;18:3496–508.
Yoo S, Krill SL, Wang Z, Telang C. Miscibility/stability considerations in binary solid dispersion systems composed of functional excipients towards the design of multi-component amorphous systems. J Pharm Sci. 2009;98:4711–23.
Wang X, Michoel A, Van den Mooter G. Solid state characteristics of ternary solid dispersions composed of PVP VA64, Myrj 52 and itraconazole. Int J Pharm. 2005;303:54–61.
Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–29.
Wang F, Yu W, Popescu C, Ibrahim AA, Yu D, Pearson R, et al. Cholecalciferol complexation with hydroxypropyl-β-cyclodextrin (HPBCD) and its molecular dynamics simulation. Pharm Dev Technol. 2022;27:389–98.
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules. 2018;23:1161.
Loftsson T. Increasing the cyclodextrin complexation of drugs and drug biovailability through addition of water-soluble polymers. Pharmazie. 1998;53:733–40.
Rowe RC, Sheskey P, Quinn M. Handbook of Pharmaceutical excipients. Ninth edition. Libros Digitales-Pharmaceutical Press; 2009.
Bhujbal SV, Mitra B, Jain U, Gong Y, Agrawal A, Karki S, et al. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm Sin B. 2021;11:2505–36.
Thakral S, Thakral NK, Majumdar DK. Eudragit®: a technology evaluation. Expert Opin Drug Deliv. 2013;10:131–49.
Duggirala NK, Li J, Kumar NSK, Gopinath T, Suryanarayanan R. A supramolecular synthon approach to design amorphous solid dispersions with exceptional physical stability. Chem Commun. 2019;55:5551–4.
Attia MS, Elshahat A, Hamdy A, Fathi AM, Emad-Eldin M, Ghazy F-ES, et al. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: recent advances in formulation strategies and pharmaceutical product features. J Drug Deliv Sci Technol. 2023;84:104519.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:1–10.
Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14:1691–8.
Bhardwaj SP, Arora KK, Kwong E, Templeton A, Clas S-D, Suryanarayanan R. Mechanism of amorphous itraconazole stabilization in polymer solid dispersions: role of molecular mobility. Mol Pharm. 2014;11:4228–37.
Mohapatra S, Samanta S, Kothari K, Mistry P, Suryanarayanan R. Effect of polymer molecular weight on the crystallization behavior of indomethacin amorphous solid dispersions. Cryst Growth Des. 2017;17:3142–50.
Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin–PVP solid dispersion obtained by spray drying. Int J Pharm. 2004;271:281–6.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272:1–10.
Chen H, Pui Y, Liu C, Chen Z, Su C-C, Hageman M, et al. Moisture-induced amorphous phase separation of amorphous solid dispersions: molecular mechanism, microstructure, and its impact on dissolution performance. J Pharm Sci. 2018;107:317–26.
Rumondor ACF, Taylor LS. Effect of polymer hygroscopicity on the phase behavior of amorphous solid dispersions in the presence of moisture. Mol Pharm. 2010;7:477–90.
Knopp MM, Wendelboe J, Holm R, Rades T. Effect of amorphous phase separation and crystallization on the in vitro and in vivo performance of an amorphous solid dispersion. Eur J Pharm Biopharm. 2018;130:290–5.
Kapourani A, Chatzitheodoridou M, Valkanioti V, Manioudaki A-E, Bikiaris ND, Barmpalexis P. Evaluating the effect of kosmotropic inorganic salts in the in vitro dissolution behavior of tablets containing amorphous indomethacin-polyvinylpyrrolidone solid dispersions. J Drug Deliv Sci Technol. 2022;72: 103421.
Rask MB, Knopp MM, Olesen NE, Holm R, Rades T. Influence of PVP/VA copolymer composition on drug–polymer solubility. Eur J Pharm Sci. 2016;85:10–7.
Knopp MM, Nguyen JH, Mu H, Langguth P, Rades T, Holm R. Influence of copolymer composition on in vitro and in vivo performance of celecoxib-PVP/VA amorphous solid dispersions. AAPS J. 2016;18:416–23.
Saboo S, Taylor LS. Water-induced phase separation of miconazole-poly (vinylpyrrolidone-co-vinyl acetate) amorphous solid dispersions: insights with confocal fluorescence microscopy. Int J Pharm. 2017;529:654–66.
Xi H, Ren J, Novak JM, Kemp E, Johnson G, Klinzing G, et al. The effect of inorganic salt on disintegration of tablets with high loading of amorphous solid dispersion containing copovidone. Pharm Res. 2020;37:70.
Pham AT, Lee PI. Probing the mechanisms of drug release from hydroxypropylmethyl cellulose matrices. Pharm Res. 1994;11:1379–84.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2012;64:163–74.
Xie T, Taylor LS. Dissolution performance of high drug loading celecoxib amorphous solid dispersions formulated with polymer combinations. Pharm Res. 2016;33:739–50.
Jansen PJ, Oren PL, Kemp CA, Maple SR, Baertschi SW. Characterization of impurities formed by interaction of duloxetine HCl with enteric polymers hydroxypropyl methylcellulose acetate succinate and hydroxypropyl methylcellulose phthalate. J Pharm Sci. 1998;87:81–5.
Yu D, Fiddler F, Ibrahim A, Sanedrin R, Tremblay H, Hoag SW. Surface characterization as a tool for identifying the factors affecting the dissolution rate of amorphous solid dispersion tablets. AAPS PharmSciTech. 2022;23:282.
Solanki NG, Lam K, Tahsin M, Gumaste SG, Shah AV, Serajuddin ATM. Effects of surfactants on itraconazole-HPMCAS solid dispersion prepared by hot-melt extrusion I: miscibility and drug release. J Pharm Sci. 2019;108:1453–65.
Ojo AT, Ma C, Lee PI. Elucidating the effect of crystallization on drug release from amorphous solid dispersions in soluble and insoluble carriers. Int J Pharm. 2020;591: 120005.
Yu D, Li J, Wang H, Pan H, Li T, Bu T, et al. Role of polymers in the physical and chemical stability of amorphous solid dispersion: a case study of carbamazepine. Eur J Pharm Sci. 2022;169: 106086.
Adhikari A, Polli JE. Characterization of grades of HPMCAS spray dried dispersions of itraconazole based on supersaturation kinetics and molecular interactions impacting formulation performance. Pharm Res. 2020;37:192.
Higashi K, Hayashi H, Yamamoto K, Moribe K. The effect of drug and EUDRAGIT® S 100 miscibility in solid dispersions on the drug and polymer dissolution rate. Int J Pharm. 2015;494:9–16.
Li J, Duggirala NK, Kumar NSK, Su Y, Suryanarayanan R. Design of ternary amorphous solid dispersions for enhanced dissolution of drug combinations. Mol Pharm. 2022;19:2950–61.
Doreth M, Löbmann K, Grohganz H, Holm R, Lopez de Diego H, Rades T, et al. Glass solution formation in water - in situ amorphization of naproxen and ibuprofen with Eudragit® E PO. J Drug Deliv Sci Technol. 2016;34:32–40.
Lubach JW, Hau J. Solid-state NMR investigation of drug-excipient interactions and phase behavior in indomethacin-eudragit E amorphous solid dispersions. Pharm Res. 2018;35:65.
Sun M, Li B, Li Y, Liu Y, Liu Q, Jiang H, et al. Experimental observations and dissipative particle dynamic simulations on microstructures of pH-sensitive polymer containing amorphous solid dispersions. Int J Pharm. 2017;517:185–95.
Wang Z, Lou H, Dening TJ, Hageman MJ. Biorelevant dissolution method considerations for the appropriate evaluation of amorphous solid dispersions: are two stages necessary? J Pharm Sci. 2023;112:1089–107.
Yao X, Kim S, Gui Y, Chen Z, Yu J, Jones KJ, et al. Amorphous drug–polymer salt with high stability under tropical conditions and fast dissolution: the challenging case of lumefantrine-PAA. J Pharm Sci. 2021;110:3670–7.
Gui Y, McCann EC, Yao X, Li Y, Jones KJ, Yu L. Amorphous drug–polymer salt with high stability under tropical conditions and fast dissolution: the case of clofazimine and poly(acrylic acid). Mol Pharm. 2021;18:1364–72.
Song Y, Yang X, Chen X, Nie H, Byrn S, Lubach JW. Investigation of drug–excipient interactions in lapatinib amorphous solid dispersions using solid-state NMR spectroscopy. Mol Pharm. 2015;12:857–66.
Nie H, Su Y, Zhang M, Song Y, Leone A, Taylor LS, et al. Solid-state spectroscopic investigation of molecular interactions between clofazimine and hypromellose phthalate in amorphous solid dispersions. Mol Pharm. 2016;13:3964–75.
Bhujbal SV, Pathak V, Zemlyanov DY, Taylor LS, Zhou Q (Tony). Physical stability and dissolution of lumefantrine amorphous solid dispersions produced by spray anti-solvent precipitation. J Pharm Sci. 2021;110:2423–31.
Alam MA, Al-Jenoobi FI, Al-mohizea AM. Commercially bioavailable proprietary technologies and their marketed products. Drug Discov Today. 2013;18:936–49.
Lavra ZMM, Pereira de Santana D, Ré MI. Solubility and dissolution performances of spray-dried solid dispersion of Efavirenz in Soluplus. Drug Dev Ind Pharm. 2017;43:42–54.
Sarpal K, Munson EJ. Amorphous solid dispersions of felodipine and nifedipine with Soluplus®: drug-polymer miscibility and intermolecular interactions. J Pharm Sci. 2021;110:1457–69.
Sarpal K, Delaney S, Zhang GGZ, Munson EJ. Phase behavior of amorphous solid dispersions of felodipine: homogeneity and drug–polymer interactions. Mol Pharm. 2019;16:4836–51.
Xie T, Taylor LS. Improved release of celecoxib from high drug loading amorphous solid dispersions formulated with polyacrylic acid and cellulose derivatives. Mol Pharm. 2016;13:873–84.
Xie T, Gao W, Taylor LS. Impact of eudragit EPO and hydroxypropyl methylcellulose on drug release rate, supersaturation, precipitation outcome and redissolution rate of indomethacin amorphous solid dispersions. Int J Pharm. 2017;531:313–23.
Pawar J, Tayade A, Gangurde A, Moravkar K, Amin P. Solubility and dissolution enhancement of efavirenz hot melt extruded amorphous solid dispersions using combination of polymeric blends: a QbD approach. Eur J Pharm Sci. 2016;88:37–49.
Butreddy A, Sarabu S, Almutairi M, Ajjarapu S, Kolimi P, Bandari S, et al. Hot-melt extruded hydroxypropyl methylcellulose acetate succinate based amorphous solid dispersions: impact of polymeric combinations on supersaturation kinetics and dissolution performance. Int J Pharm. 2022;615: 121471.
Newman A, Zografi G. Considerations in the development of physically stable high drug load API-polymer amorphous solid dispersions in the glassy state. J Pharm Sci. 2023;112:8–18.
Lin X, Hu Y, Liu L, Su L, Li N, Yu J, et al. Physical stability of amorphous solid dispersions: a physicochemical perspective with thermodynamic, kinetic and environmental aspects. Pharm Res. 2018;35:125.
Hiew TN, Taylor LS. Combining drug salt formation with amorphous solid dispersions–a double edged sword. J Control Release. 2022;352:47–60.
Van DT, Nguyen HT, Taylor LS. Combining enabling formulation strategies to generate supersaturated solutions of delamanid: in situ salt formation during amorphous solid dispersion fabrication for more robust release profiles. Eur J Pharm Biopharm. 2022;174:131–43.
Fung MH, Suryanarayanan R. Effect of organic acids on molecular mobility, physical stability, and dissolution of ternary ketoconazole spray-dried dispersions. Mol Pharm. 2019;16:41–8.
Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59:603–16.
Mukesh S, Joshi P, Bansal AK, Kashyap MC, Mandal SK, Sathe V, et al. Amorphous salts solid dispersions of celecoxib: enhanced biopharmaceutical performance and physical stability. Mol Pharm. 2021;18:2334–48.
Liu X, Zhou L, Zhang F. Reactive melt extrusion to improve the dissolution performance and physical stability of naproxen amorphous solid dispersions. Mol Pharm. 2017;14:658–73.
Löbmann K, Laitinen R, Grohganz H, Gordon KC, Strachan C, Rades T. Coamorphous drug systems: enhanced physical stability and dissolution rate of indomethacin and naproxen. Mol Pharm. 2011;8:1919–28.
Allesø M, Chieng N, Rehder S, Rantanen J, Rades T, Aaltonen J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: amorphous naproxen–cimetidine mixtures prepared by mechanical activation. J Control Release. 2009;136:45–53.
Liu J, Grohganz H, Löbmann K, Rades T, Hempel N-J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics. 2021;13:389.
Haser A, Cao T, Lubach JW, Zhang F. In situ salt formation during melt extrusion for improved chemical stability and dissolution performance of a meloxicam–copovidone amorphous solid dispersion. Mol Pharm. 2018;15:1226–37.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33:1043–57.
Newman A, Zografi G. Perspectives on the wetting of solids in pharmaceutical systems. Pharm Res. 2023. https://doi.org/10.1007/s11095-023-03491-3.
Yao X, Benson EG, Gui Y, Stelzer T, Zhang GGZ, Yu L. Surfactants accelerate crystallization of amorphous nifedipine by similar enhancement of nucleation and growth independent of hydrophilic–lipophilic balance. Mol Pharm. 2022;19:2343–50.
LaFountaine JS, McGinity JW, Williams RO. Challenges and strategies in thermal processing of amorphous solid dispersions: a review. AAPS PharmSciTech. 2016;17:43–55.
Bhanderi A, Bari F, Al-Obaidi H. Evaluation of the impact of surfactants on miscibility of griseofulvin in spray dried amorphous solid dispersions. J Drug Deliv Sci Technol. 2021;64: 102606.
Gumaste SG, Gupta SS, Serajuddin ATM. Investigation of polymer-surfactant and polymer-drug-surfactant miscibility for solid dispersion. AAPS J. 2016;18:1131–43.
Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: stability testing of selected solid dispersions. Pharm Res. 2006;23:1928–36.
Yang R, Zhang GG, Zemlyanov DY, Purohit HS, Taylor LS. Drug release from surfactant-containing amorphous solid dispersions: mechanism and role of surfactant in release enhancement. Pharm Res. 2023. https://doi.org/10.1007/s11095-023-03502-3.
Meng F, Ferreira R, Zhang F. Effect of surfactant level on properties of celecoxib amorphous solid dispersions. J Drug Deliv Sci Technol. 2019;49:301–7.
Schittny A, Philipp-Bauer S, Detampel P, Huwyler J, Puchkov M. Mechanistic insights into effect of surfactants on oral bioavailability of amorphous solid dispersions. J Control Release. 2020;320:214–25.
Saboo S, Bapat P, Moseson D, Kestur U, Taylor L. Exploring the role of surfactants in enhancing drug release from amorphous solid dispersions at higher drug loadings. Pharmaceutics. 2021;13:735.
Chen Y, Wang S, Wang S, Liu C, Su C, Hageman M, et al. Sodium lauryl sulfate competitively interacts with HPMC-AS and consequently reduces oral bioavailability of posaconazole/HPMC-AS amorphous solid dispersion. Mol Pharm. 2016;13:2787–95.
Pui Y, Chen Y, Chen H, Wang S, Liu C, Tonnis W, et al. Maintaining supersaturation of nimodipine by PVP with or without the presence of sodium lauryl sulfate and sodium taurocholate. Mol Pharm. 2018;15:2754–63.
Deshpande TM, Shi H, Pietryka J, Hoag SW, Medek A. Investigation of polymer/surfactant interactions and their impact on itraconazole solubility and precipitation kinetics for developing spray-dried amorphous solid dispersions. Mol Pharm. 2018;15:962–74.
Wang S, Liu C, Chen H, Zhu A (Donghua), Qian F. Impact of surfactants on polymer maintained nifedipine supersaturation in aqueous solution. Pharm Res. 2020;37:113.
Liu C, Chen Z, Chen Y, Lu J, Li Y, Wang S, et al. Improving oral bioavailability of sorafenib by optimizing the “spring” and “parachute” based on molecular interaction mechanisms. Mol Pharm. 2016;13:599–608.
Tung N-T, Tran C-S, Nguyen T-L, Pham T-M-H, Chi S-C, Nguyen H-A, et al. Effect of surfactant on the in vitro dissolution and the oral bioavailability of a weakly basic drug from an amorphous solid dispersion. Eur J Pharm Sci. 2021;162:105836.
Chen J, Ormes JD, Higgins JD, Taylor LS. Impact of surfactants on the crystallization of aqueous suspensions of celecoxib amorphous solid dispersion spray dried particles. Mol Pharm. 2015;12:533–41.
Correa-Soto CE, Gao Y, Indulkar AS, Zhang GGZ, Taylor LS. Role of surfactants in improving release from higher drug loading amorphous solid dispersions. Int J Pharm. 2022;625: 122120.
Yu J, Li Y, Yao X, Que C, Huang L, Hui H-W, et al. Surface enrichment of surfactants in amorphous drugs: an x-ray photoelectron spectroscopy study. Mol Pharm. 2022;19:654–60.
Guo Y, Sun CC. Pharmaceutical lauryl sulfate salts: prevalence, formation rules, and formulation implications. Mol Pharm. 2022;19:432–9.
Guo Y, Mishra MK, Wang C, Sun CC. Crystallographic and energetic insights into reduced dissolution and physical stability of a drug–surfactant salt: the case of norfloxacin lauryl sulfate. Mol Pharm. 2019;17(2):579–87.
Guo Y, Wang C, Dun J, Du L, Hawley M, Sun CC. Mechanism for the reduced dissolution of ritonavir tablets by sodium lauryl sulfate. J Pharm Sci. 2019;108:516–24.
Li J, Wu Y. Lubricants in pharmaceutical solid dosage forms. Lubricants. 2014;2:21–43.
Démuth B, Nagy ZK, Balogh A, Vigh T, Marosi G, Verreck G, et al. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int J Pharm. 2015;486:268–86.
Thakral NK, Mohapatra S, Stephenson GA, Suryanarayanan R. Compression-induced crystallization of amorphous indomethacin in tablets: characterization of spatial heterogeneity by two-dimensional x-ray diffractometry. Mol Pharm. 2015;12:253–63.
Koranne S, Lalge R, Suryanarayanan R. Modulation of microenvironmental acidity: a strategy to mitigate salt disproportionation in drug product environment. Mol Pharm. 2020;17:1324–34.
Nie H, Xu W, Ren J, Taylor LS, Marsac PJ, John CT, et al. Impact of metallic stearates on disproportionation of hydrochloride salts of weak bases in solid-state formulations. Mol Pharm. 2016;13:3541–52.
Duggirala NK, Vyas A, Krzyzaniak JF, Arora KK, Suryanarayanan R. Mechanistic insight into caffeine–oxalic cocrystal dissociation in formulations: role of excipients. Mol Pharm. 2017;14:3879–87.
Koranne S, Sahoo A, Krzyzaniak JF, Luthra S, Arora KK, Suryanarayanan R. Challenges in transitioning cocrystals from bench to bedside: dissociation in prototype drug product environment. Mol Pharm. 2018;15:3297–307.
Yu D, Nie H. Evaluation of alternative metallic stearates as lubricants in pharmaceutical tablet formulation. AAPS PharmSciTech. 2022;23:200.
Démuth B, Farkas A, Balogh A, Bartosiewicz K, Kállai-Szabó B, Bertels J, et al. Lubricant-induced crystallization of itraconazole from tablets made of electrospun amorphous solid dispersion. J Pharm Sci. 2016;105:2982–8.
Démuth B, Galata DL, Szabó E, Nagy B, Farkas A, Balogh A, et al. Investigation of deteriorated dissolution of amorphous itraconazole: description of incompatibility with magnesium stearate and possible solutions. Mol Pharm. 2017;14:3927–34.
Démuth B, Galata DL, Balogh A, Szabó E, Nagy B, Farkas A, et al. Application of hydroxypropyl methylcellulose as a protective agent against magnesium stearate induced crystallization of amorphous itraconazole. Eur J Pharm Sci. 2018;121:301–8.
Hughey JR, Keen JM, Miller DA, Kolter K, Langley N, McGinity JW. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus®-based solid dispersions. Eur J Pharm Sci. 2013;48:758–66.
Takano R, Maurer R, Jacob L, Stowasser F, Stillhart C, Page S. Formulating amorphous solid dispersions: impact of inorganic salts on drug release from tablets containing itraconazole-HPMC extrudate. Mol Pharm. 2020;17:2768–78.
Zhang Y, Cremer P. Interactions between macromolecules and ions: the Hofmeister series. Curr Opin Chem Biol. 2006;10:658–63.
Author information
Authors and Affiliations
Contributions
The writing, reviewing, and editing of the review paper are assigned to the authors as follows:
Jinghan Li: introduction, sections of polymer combination, counterions, surfactants, and inorganic salts.
Yihan Wang: sections of the commonly used polymers in ASDs.
Dongyue Yu: writing of abstract, introduction and conclusion, sections of surfactants, MgSt, inorganic salts, and overall reviewing and editing of the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Wang, Y. & Yu, D. Effects of Additives on the Physical Stability and Dissolution of Polymeric Amorphous Solid Dispersions: a Review. AAPS PharmSciTech 24, 175 (2023). https://doi.org/10.1208/s12249-023-02622-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02622-8