Abstract
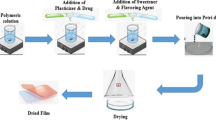
Asenapine, an atypical antipsychotic agent, has been approved for the acute and maintenance treatment of schizophrenia and manic episodes of bipolar disorder. However, the extensive hepatic metabolism limits its oral bioavailability. Therefore, the objective of the current investigation was to develop sublingual film containing asenapine to enhance the therapeutic efficacy. Sublingual films containing asenapine were fabricated using polyethylene oxide and hydroxypropyl methylcellulose by solvent casting method. Design of experiment was used as a statistical tool to optimize the proportion of the film-forming polymers in order to establish the critical quality attributes of the drug formulation. The process was studied in detail by assessing risk of each step as well as parameters and material attributes to reduce the risk to a minimum. A control strategy was defined to ensure manufacture of films according to the target product profile by evaluation of intermediate quality attributes at the end of each process step. Results of optimized formulations showed rapid disintegration, adequate folding endurance, good percentage elongation, tensile strength, and viscosity. Besides, the results from the in vitro dissolution/ex vivo permeation studies showed rapid dissolution (100% in 6 min) and higher asenapine permeation (~ 80% in 90 min) through the sublingual epithelium. In vivo study indicates greater asenapine absorption (31.18 ± 5.01% of administered dose) within 5 min and was comparable with marketed formulation. In summary, the designing plan to develop asenapine formulation was successfully achieved with desired characteristics of the delivery tool for sublingual administration.











Similar content being viewed by others
References
Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–34. https://doi.org/10.2147/ndt.S225643.
Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1. https://doi.org/10.1186/s40345-019-0160-1.
Kumbhar SA, Kokare CR, Shrivastava B, Gorain B. Screening of nanoemulsion components for asenapine maleate using validated RP-HPLC method. Ann Pharm Fr. 2020;78(5):379–87. https://doi.org/10.1016/j.pharma.2020.04.005.
Marazziti D, Piccinni A, Baroni S, Mungai F, Presta S, Mucci F, et al. Current trends on antipsychotics: focus on asenapine. Curr Med Chem. 2016;23(21):2204–16. https://doi.org/10.2174/0929867323666160525115014.
Managuli RS, Wang JT, Faruqu FN, Kushwah V, Raut SY, Shreya AB, et al. Asenapine maleate-loaded nanostructured lipid carriers: optimization and in vitro, ex vivo and in vivo evaluations. Nanomedicine (Lond). 2019;14(7):889–910. https://doi.org/10.2217/nnm-2018-0289.
Managuli RS, Wang JT, Faruqu FM, Pandey A, Jain S, Al-Jamal KT, et al. Surface engineered nanoliposomal platform for selective lymphatic uptake of asenapine maleate: in vitro and in vivo studies. Mater Sci Eng, C Mater Biol Appl. 2020;109: 110620. https://doi.org/10.1016/j.msec.2019.110620.
Patel M, Mundada V, Sawant K. Enhanced intestinal absorption of asenapine maleate by fabricating solid lipid nanoparticles using TPGS: elucidation of transport mechanism, permeability across Caco-2 cell line and in vivo pharmacokinetic studies. Artif Cells Nanomed Biotechnol. 2019;47(1):144–53. https://doi.org/10.1080/21691401.2018.1546186.
Kumbhar SA, Kokare CR, Shrivastava B, Gorain B, Choudhury H. Preparation, characterization, and optimization of asenapine maleate mucoadhesive nanoemulsion using Box-Behnken design: in vitro and in vivo studies for brain targeting. Int J Pharm. 2020;586: 119499. https://doi.org/10.1016/j.ijpharm.2020.119499.
Zhou M, Derakhshanian S, Rath A, Bertrand S, DeGraw C, Barlow R, et al. Asenapine transdermal patch for the management of schizophrenia. Psychopharmacol Bull. 2020;50(4):60–82.
Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–405. https://doi.org/10.1021/mp100149j.
Nair AB, Al-Dhubiab BE, Shah J, Jacob S, Saraiya V, Attimarad M, et al. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm J. 2020;28(2):201–9. https://doi.org/10.1016/j.jsps.2019.11.022.
Kumria R, Al-Dhubiab BE, Shah J, Nair AB. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J Pharm Innov. 2018;13(2):133–43. https://doi.org/10.1007/s12247-018-9312-6.
Pinto S, Pintado ME, Sarmento B. In vivo, ex vivo and in vitro assessment of buccal permeation of drugs from delivery systems. Expert Opin Drug Deliv. 2020;17(1):33–48. https://doi.org/10.1080/17425247.2020.1699913.
Hanif M, Zaman M, Chaurasiya V. Polymers used in buccal film: a review. Des Monomers Polym. 2015;18(2):105–11. https://doi.org/10.1080/15685551.2014.971389.
Laffleur F. Mucoadhesive polymers for buccal drug delivery. Drug Dev Ind Pharm. 2014;40(5):591–8. https://doi.org/10.3109/03639045.2014.892959.
Ma L, Deng L, Chen J. Applications of poly(ethylene oxide) in controlled release tablet systems: a review. Drug Dev Ind Pharm. 2014;40(7):845–51. https://doi.org/10.3109/03639045.2013.831438.
Salehi S, Boddohi S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog Biomater. 2017;6(4):175–87. https://doi.org/10.1007/s40204-017-0077-7.
Prodduturi S, Manek RV, Kolling WM, Stodghill SP, Repka MA. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J Pharm Sci. 2005;94(10):2232–45. https://doi.org/10.1002/jps.20437.
Nair A, Gupta R, Vasanti S. In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm Dev Technol. 2007;12(6):621–5. https://doi.org/10.1080/10837450701563277.
SreeHarsha N, Hiremath JG, Sarudkar S, Attimarad M, Al-Dhubiab B, Nair AB, et al. Spray dried amorphous form of simvastatin: preparation and evaluation of the buccal tablet. Indian J Pharm Educ Res. 2020;54:46–54.
Managuli RS, Kumar L, Chonkar AD, Shirodkar RK, Lewis S, Koteshwara KB, et al. Development and validation of a stability-indicating RP-HPLC method by a statistical optimization process for the quantification of asenapine maleate in lipidic nanoformulations. J Chromatogr Sci. 2016;54(8):1290–300. https://doi.org/10.1093/chromsci/bmw062.
Osborne DW. Impact of quality by design on topical product excipient suppliers, part I: a drug manufacturer's perspective. 2016.
Namjoshi S, Dabbaghi M, Roberts MS, Grice JE, Mohammed Y. Quality by design: development of the quality target product profile (QTPP) for semisolid topical products. Pharmaceutics. 2020;12(3). https://doi.org/10.3390/pharmaceutics12030287.
Food, Administration D, editors. ICH Q8 (R2) pharmaceutical development. workshop: quality by design in pharmaceutical; 2009.
Food U, Administration D. Quality by design for ANDAs: an example for immediate-release dosage forms. US Department of Health and Human Service (FDA, Rockville, MD, 2012). 2012.
Dangre PV, Phad RD, Surana SJ, Chalikwar SS. Quality by Design (QbD) Assisted fabrication of fast dissolving buccal film for clonidine hydrochloride: exploring the quality attributes. Adv Polym Technol. 2019;2019:3682402. https://doi.org/10.1155/2019/3682402.
Borges AF, Silva C, Coelho JF, Simões S. Oral films: current status and future perspectives: I - galenical development and quality attributes. J Control Release. 2015;206:1–19. https://doi.org/10.1016/j.jconrel.2015.03.006.
Myers GL, Hilbert SD, Boone BJ, Bogue BA, Sanghvi P, Hariharan M. Sublingual and buccal film compositions. Google Patents; 2013.
Akrawi SH, Gorain B, Nair AB, Choudhury H, Pandey M, Shah JN, et al. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics. 2020;12(9). https://doi.org/10.3390/pharmaceutics12090893.
Chaudhary S, Nair AB, Shah J, Gorain B, Jacob S, Shah H, et al. Enhanced solubility and bioavailability of dolutegravir by solid dispersion method: in vitro and in vivo evaluation—a potential approach for HIV therapy. AAPS PharmSciTech. 2021;22(3):127. https://doi.org/10.1208/s12249-021-01995-y.
Hoffmann EM, Breitenbach A, Breitkreutz J. Advances in orodispersible films for drug delivery. Expert Opin Drug Deliv. 2011;8(3):299–316. https://doi.org/10.1517/17425247.2011.553217.
Nair AB, Shah J, Jacob S, Al-Dhubiab BE, Patel V, Sreeharsha N, et al. Development of mucoadhesive buccal film for rizatriptan: in vitro and in vivo evaluation. Pharmaceutics. 2021;13(5). https://doi.org/10.3390/pharmaceutics13050728.
Singh TP, Singh RK, Shah JN, Mehta TA. Mucoadhesive bilayer buccal patches of Verapamil hydrochloride: formulation development and characterization. Int J Pharm Pharm Sci. 2014;6(4):234–41.
Garsuch V, Breitkreutz J. Novel analytical methods for the characterization of oral wafers. Eur J Pharm Biopharm. 2009;73(1):195–201. https://doi.org/10.1016/j.ejpb.2009.05.010.
Nair AB, Kumria R, Harsha S, Attimarad M, Al-Dhubiab BE, Alhaider IA. In vitro techniques to evaluate buccal films. J Control Release. 2013;166(1):10–21. https://doi.org/10.1016/j.jconrel.2012.11.019.
Renuka M, Nishadh P, Jigar S, Tejal M. Mucoadhesive wound healing film of doxycycline hydrochloride. Int J Drug Dev Res. 2012;4:128–40.
Shehata TM, Nair AB, Al-Dhubiab BE, Shah J, Jacob S, Alhaider IA, et al. Vesicular emulgel based system for transdermal delivery of insulin: factorial design and in vivo evaluation. Appl Sci. 2020;10(15):5341.
Jug M, Hafner A, Lovrić J, Kregar ML, Pepić I, Vanić Ž, et al. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J Pharm Biomed Anal. 2018;147:350–66. https://doi.org/10.1016/j.jpba.2017.06.072.
Nair AB, Al-Dhubiab BE, Shah J, Vimal P, Attimarad M, Harsha S. Development and evaluation of palonosetron loaded mucoadhesive buccal films. J Drug Deliv Sci Technol. 2018;47:351–8. https://doi.org/10.1016/j.jddst.2018.08.014.
Guideline IHT. Validation of analytical procedures: text and methodology. Q2 (R1). 2005;1(20):05.
Shah JN, Shah KN, Mehta TA. Hydroxy propyl β-cyclodextrin complexation of promethazine hydrochloride for the formulation of fast dissolving sublingual film: in vitro and in vivo evaluation. J Pharm Investig. 2015;45(1):91–9.
Panraksa P, Udomsom S, Rachtanapun P, Chittasupho C, Ruksiriwanich W, Jantrawut P. Hydroxypropyl methylcellulose E15: a hydrophilic polymer for fabrication of orodispersible film using syringe extrusion 3D printer. Polymers. 2020;12(11). https://doi.org/10.3390/polym12112666.
Sadeghi F, Shahabi M, Afrasiabi H. Comparison of physicomechanical properties of films prepared from organic solutions and aqueous dispersion of Eudragit RL. Daru. 2011;19(2):100–6.
Al-Dhubiab BE, Nair AB, Kumria R, Attimarad M, Harsha S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Delivery. 2016;23(7):2154–62. https://doi.org/10.3109/10717544.2014.948644.
Nair AB, Gupta S, Al-Dhubiab BE, Jacob S, Shinu P, Shah J, et al. Effective therapeutic delivery and bioavailability enhancement of pioglitazone using drug in adhesive transdermal patch. Pharmaceutics. 2019;11(7). https://doi.org/10.3390/pharmaceutics11070359.
Jacob S, Nair AB. An updated overview with simple and practical approach for developing in vitro-in vivo correlation. Drug Dev Res. 2018;79(3):97–110. https://doi.org/10.1002/ddr.21427.
Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–63. https://doi.org/10.1021/acs.chemrev.5b00346.
Kavanagh N, Corrigan OI. Swelling and erosion properties of hydroxypropylmethylcellulose (Hypromellose) matrices–influence of agitation rate and dissolution medium composition. Int J Pharm. 2004;279(1–2):141–52. https://doi.org/10.1016/j.ijpharm.2004.04.016.
Viridén A, Larsson A, Wittgren B. The effect of substitution pattern of HPMC on polymer release from matrix tablets. Int J Pharm. 2010;389(1–2):147–56. https://doi.org/10.1016/j.ijpharm.2010.01.029.
Nair AB, Shah J, Aljaeid BM, Al-Dhubiab BE, Jacob S. Gellan Gum-based hydrogel for the transdermal delivery of nebivolol: optimization and evaluation. Polymers. 2019;11(10). https://doi.org/10.3390/polym11101699.
Shah J, Nair AB, Jacob S, Patel RK, Shah H, Shehata TM, et al. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics. 2019;11(5). https://doi.org/10.3390/pharmaceutics11050230.
Kalia V, Garg T, Rath G, Goyal AK. Development and evaluation of a sublingual film of the antiemetic granisetron hydrochloride. Artif Cells Nanomed Biotechnol. 2016;44(3):842–6. https://doi.org/10.3109/21691401.2014.984303.
Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79(8):373–82. https://doi.org/10.1002/ddr.21461.
Avachat AM, Kapure SS. Asenapine maleate in situ forming biodegradable implant: an approach to enhance bioavailability. Int J Pharm. 2014;477(1–2):64–72. https://doi.org/10.1016/j.ijpharm.2014.10.006.
Singh SK, Hidau MK, Gautam S, Gupta K, Singh KP, Singh SK, et al. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: pharmacokinetic and teratogenic assessment. Int J Biol Macromol. 2018;108:1092–100. https://doi.org/10.1016/j.ijbiomac.2017.11.031.
Shreya AB, Managuli RS, Menon J, Kondapalli L, Hegde AR, Avadhani K, et al. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: in vitro and in vivo performance evaluations. J Liposome Res. 2016;26(3):221–32. https://doi.org/10.3109/08982104.2015.1098659.
Acknowledgements
The authors acknowledge Sun Pharmaceuticals Industries Ltd, Vadodara, India, for providing drug and polymers.
Author information
Authors and Affiliations
Contributions
Conceptualization: Rahil Dalal, Jigar Shah, Bapi Gorain, Shery Jacob, and Anroop B. Nair; data curation: Rahil Dalal, Jigar Shah, Bapi Gorain, Hira Choudhury, and Shery Jacob; formal analysis: Rahil Dalal, Jigar Shah, Hira Choudhury, Shery Jacob, Tejal A. Mehta, Hiral Shah, and Anroop B. Nair; methodology: Rahil Dalal, Jigar Shah, Bapi Gorain, Hira Choudhury, Shery Jacob, Tejal A. Mehta, Hiral Shah, and Anroop B. Nair; writing—original draft preparation: Rahil Dalal, Tejal A. Mehta, and Hiral Shah; writing—review and editing: Jigar Shah, Bapi Gorain, Hira Choudhury, Shery Jacob, and Anroop B. Nair. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dalal, R., Shah, J., Gorain, B. et al. Development and Optimization of Asenapine Sublingual Film Using QbD Approach. AAPS PharmSciTech 22, 244 (2021). https://doi.org/10.1208/s12249-021-02132-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02132-5