Abstract
Background
The present research work aims to develop a Ropinirole-loaded self-Nanoemulsifying Drug Delivery system. Ropinirole has limited oral bioavailability due to substantial first-pass metabolism, which ultimately results in poor oral bioavailability and reduces plasma drug concentration and an overall reduction in therapeutic effects. Avoiding hepatic first-pass metabolism by increasing lymphatic uptake is the goal of the creation of the Ropinirole Self-NanoEmulsifying System. The solvent system for the liquid Self-NanoEmulsifying Drug Delivery System (SNEDDS) was optimized using Box-Behnken Design, where the concentration of oil(X1), surfactant (X2), and co-surfactant(X3) were taken as independent variables. The formulated liquid SNEDDS were then converted into solid SNEDDS by the adsorption method for improving patient compliance.
Results
The obtained mean droplet size of the formulated SNEDDS was 96.71 nm, and the rate of emulsification was 22 s. Liquid SNEDDS was converted into solid SNEDDS using Syloid 244 FP as adsorbent. Scanning Electron Microscopy (SEM) study shows well-separated particles adsorbed on Syloid 244 FP. In vitro drug release studies show better release from solid and liquid SNEDDS when compared to pure suspension.
Conclusions
Ropinirole-loaded SNEDDS can be a better option for reducing the extensive first-pass metabolism associated with Ropinirole.
Similar content being viewed by others
Background
The oral route has become the primary drug distribution route for chronic care of many diseases because it has a high degree of conformity for patients. However, due to the elevated lipophilicity of the medication itself, oral delivery of 50 percent of the drug compounds is hindered [1]. About 40 percent of new drug candidates have poor water solubility, which is a difficulty in designing the best type of oral solid dosage in terms of formulation design and bioavailability. Many methods have been used to resolve these problems by adjusting the solubility or preserving the substance in dissolved form during gastric transit time. It was also suggested to use formulation techniques such as solid dispersion, complexation with cyclodextrins, and micronization to increase solubility. For solid dispersion, costly facilities and procedures are used for the freeze-drying or spray-drying methods, which result in high product costs. Instead, conventional solvent-evaporation approaches could have been used, but dealing with co-precipitates with high viscosities is also challenging. Drugs not soluble in both aqueous and organic solvents cannot be complexed using cyclodextrins.
SNEDDS can overcome these drawbacks as drugs with low aqueous solubility are soluble in their lipophilic phase.
SNEDDS is an isotropic oil, surfactant, and co-surfactant combination that creates fine oil in water nanoemulsion once it encounters the aqueous fluid present in GIT. Recently, there is an increased interest in the formulation of poorly water-soluble pharmaceuticals in lipids due to the possibility that oral bioavailability of poorly water-soluble medications may increase when co-administered with meals high in fat. Moreover, liquid SNEDDS can be converted to solid SNEDDS by less costly techniques like adsorption. Drugs administered at a very high dose are not appropriate for SNEDDS unless they are well solubilized in at least one of the lipophilic excipients of SNEDDS. For this reason, lipophilic drugs can be a good candidate for SNEDDS formulation. The drug must be physically and chemically stable in the formulation, and during the shelf life of the SNEDDS formulation, the drug release pattern must remain constant [2]. These attributes of SNEDDS as a drug delivery system make Ropinirole a good candidate for SNEDDS formulation.
Ropinirole is a prescription medication used for the treatment of movement disorders. It is a selective non-ergoline dopamine D2 receptor agonist indicated for use in treating Parkinson's disease. It can treat both early and advanced stages of Parkinson’s disease. In Parkinson's disease, there is a loss of dopamine receptors in the substantia nigra pars compacta. The slow movement, tremors, and stiff joints are its characteristics. Upon oral absorption, about 50% of the Ropinirole undergoes first-pass metabolism eliciting an absolute bioavailability of 45–55%. The liver biotransforms most of the drug into inactive metabolites. The ileum portion of the small intestine contains peyer’s patches, which possess a dense network of villi. When SNEDDS formulation encounters gastric fluid, the peristaltic movement of the stomach converts it into nano-emulsion. The nanodroplets can be directly absorbed through the Peyer’s patches and enter lymphatic circulation by avoiding first-pass metabolism.
Materials and methods
Materials
The required pharmaceutical active ingredient Ropinirole was obtained as a gift sample from Intas Pharmaceuticals Ltd. Ahmedabad. Other excipients required for the development of SNEDDS include different oils, surfactants, and co-surfactants purchased from Chemdyes Corporation. For preliminary trials, castor oil, Capmul MCM EP, Capmul MCM C8, Miglyol 812 N, Captex 200 P, and Labrafac PG were tested for selection of appropriate oil. Similarly, Transcutol HP, Acrysol EL 135, and Acconon MC8 as co-surfactants, and Span 20, Span 80, Tween 20, and Tween 80 as surfactants were also screened. Apart from these, adsorbing agents experimented on were Aerosil 300 Pharma, Syloid 244 FP, Neusilin UFL 2, Cross-povidone, and MCC for conversion of liquid SNEDDS into solid SNEDDS (Fig. 1).
Pre-formulation studies
Drug excipient compatibility study by Fourier transform infra-red spectroscopy (FTIR)
The identification of Ropinirole and interaction between Ropinirole and selected oil (Capmul MCM EP), surfactant (Tween 20), and co-surfactant (Acrysol EL 135) is studied by Fourier Transform Infra-Red Spectroscopy. FTIR spectrum observations between 400 and 4000 cm−1 were studied on Shimadzu FTIR Spectrometer.
Solubility studies of the drug in various oils, surfactants, and co-surfactants
The drug's solubility was investigated by a quantitative approach. Ropinirole was added in increments of 1 mg until it stopped dissolving in the predetermined 1 mL of solvent (oil, surfactant, or co-surfactant). The drug and solvent mixture was vortexed for 10 min and then sonicated. The amount of drug dissolved in various oils, surfactants, and co-surfactants was calculated [3].
Preliminary tests for choosing an excipient combination
Two solvents from each category of oil, surfactant, and co-surfactant with higher solubilities were chosen based on drug-solubility investigations. Capmul MCM C8 and Capmul MCM EP as oil, Tween 20 and Span 80 as surfactants, and Transcutol HP and Acrysol EL 135 as co-surfactant were selected. Miglyol 812 N, castor oil, Captex 355, Captex 200 P, and Labrafac PG in the category of oil, Acconon MC8 in the category of co-surfactant, and Span 20, Tween 40 in the category of surfactants were eliminated since they exhibited low solubility for Ropinirole. A 23 combination gives 8 preliminary trial batches for the selection of the solvent system. Each experimental batch was made by combining 1 mL of oil, 1 mL of surfactant, 1 mL of cosurfactant, and 5 mg of the medication. The obtained formulations were assessed for transparency, drug precipitation, and emulsification effectiveness as shown in Table 2.
Construction of ternary phase diagram
Various plots from the ternary plot were selected for studying the Ternary Phase, as depicted in Fig. 2. Based on this, 22 batches of solvent systems with different concentrations were produced. Its self-emulsification rate and transparency- rates were investigated. Formulations with an emulsification rate of less than 1 min and a transparency level greater than 90% were chosen to find the Self-nanoemulsifying zone [4].
Optimization of formulation: Box–Behnken design
Traditional pharmaceutical formulation design is based on the time-consuming method of modifying one variable at a time, ignoring independent variables' mutual effect. As a consequence, factorial layout may be a valuable method for evaluating the complexity of pharmaceutical formulations [4]. The formulation was optimized using the Box-Behnken design owing to its numerous advantages over the full-factorial design. A comparative study between central composite and three-level full-factorial design showed that the BBD is slightly more efficient than the central composite design and much more efficient than the three-level full factorial designs. Three-level full-factorial design is costly when the factor number is more than 2. Another advantage of the BBD is that it does not contain combinations for which all factors are simultaneously at their highest or lowest levels.
Details of independent factors, coded and uncoded levels, and design points are given in Table 1. Checkpoint batches (Table 2) were prepared to evaluate the predictability of the optimization model. Droplet size(Y1), Rate of emulsification(Y2), concentration of oil(X1), surfactant(X2), co-surfactant(X3) was selected as criteria for optimization. Each Run point was prepared by mixing respective components in a clean screw-caped plastic tube of 25 mL and mixed thoroughly by vortex mixture. Each formulation contained 5 mg of Ropinirole.
Check point batches
The predictability of the optimization model was evaluated by checkpoint batches (Table 2). Droplet size (Y1), Rate of Emulsification (Y2), Surfactant concentration (X2), Co-surfactant concentration, (X3), and Oil concentration(X1) were the criteria for optimization.
Solidification of liquid SNEDDS
Conversion of liquid SNEDDS into solid SNEDDS helps to overthrow one of the main disadvantages of conventional micro-emulsions. Liquid SNEDDS are unstable and exhibit problems in handling, storage, and stability. Moreover, administering medication in the form of oil reduces patient compliance too. The formulation of the tablet is more advantageous than that of capsules as there may be a chance that oil may interact with the shells of the capsule and can degrade them. Solid SMEDDS as tablets would be more stable than liquid SMEDDS. Also, formulation as fast dispersible tablets gives the spontaneous formation of an emulsion.
Estimation of optimum liquid: adsorbent ratio
Optimized liquid SNEDDS formulation was converted to free-flowing and compressible powder by carriers or adsorbents, including Aeroperl 300 Pharma, Syloid 244 FP, and Neusilin UFL 2 [5].
Liquid SNEDDS formulation of specified quantity was diluted with 1.5 times more amount of isopropyl alcohol in mortar. A predetermined quantity of adsorbent material was added to the mortar, and it was properly mixed with a spatula until a paste-like mass was created. This paste-like substance was heated in an oven at 50 °C for 0.5–1 h until isopropyl alcohol gets evaporated completely. The isopropyl alcohol was employed to perform efficient mixing of liquid SNEDDS with the adsorbents. Later, the volatile isopropyl alcohol evaporates and leaves a good homogeneous powder of SNEDDS.
Pre-formulation parameters of tablets like Carr’s Index, Hausner’s Ratio, and Angle of Repose were chosen as a function of optimum liquid: Adsorbent ratio. Various proportions of liquid SNEDDS to adsorbent material from 1:0.5 to 1:4 (mL:g) were investigated [6]. Powder prepared by these proportions was evaluated for pre-formulation parameters. The ideal mixture of liquid and adsorbent was determined to generate powder with the proper flowability and compressibility [7].
Ropinirole SNEDDS tablet preparation
The tablet was prepared by Direct Compression Method was used to prepare the tablet [7]. Each tablet contains 400 mg SNEDDS powder equivalent to 2 mg of Ropinirole. All components were mixed into a clean mortar and passed through a 22# sieve size. After mixing, the powder mixture was subjected to a tablet compression unit for tablet preparation (535 mg each).
Evaluation of liquid SNEDDS
Self-emulsification efficiency
The effectiveness of self-emulsification was assessed using qualitative grading techniques and visual inspection. Using the USP-II dissolution device, self-emulsifying efficiency was calculated. 1 ml of the formulation was added dropwise to 200 ml of 0.1 N HCL (37 °C). The rotating paddle was kept at a speed of 60 rotations per minute (RPM) to ensure mild agitation. After complete dispersion, the emulsion's quality and emulsification rate were assessed. A stop clock was used to visually quantify the rate of emulsification or the time needed for complete dispersion. Emulsion Appearance Qualitative grading (EAQG) for emulsion was given according to Table 3.
Evaluation of transparency
Using a UV–Visible spectrophotometer, Shimadzu 1800, the percentage of transparency was calculated after diluting the SNEDDS formulation (1 mL with 200 mL) with pure water at 650 nm. Water that has been purified was used as the blank and standard. Readings are expressed as a percentage of transmittance (%T) [8].
Droplet size determination
The Dynamic Light Scattering method was used to determine the droplet diameter. 200 mL of distilled water and 1 mL of the SNEDDS formulation were combined to create the sample while being gently stirred. The sample analyzed was used for droplet size distribution in the Malvern Zetasizer Nano S 90 after one hour [3].
In vitro drug release studies
The Dialysis Bag Method was used to study the drug release pattern of liquid SNEDDS. Distilled water was used to dilute the final optimized formulation, which contained 2 mg of ropinirole. 1 mL of nano emulsion was transferred into the dialysis bag. Dialysis tubing was made of cellulose. It is a thin polymeric membrane with consistent pore size, maximal wet strength, and compatibility with a wide range of solvents. The tubing was spun at 60 RPM while immersed in 200 mL phosphate buffer with a pH of 7.4. 1 mL of the sample was taken out at regular intervals for 2 h. The absorbance was used to calculate the amount of Ropinirole present. The sink conditions were maintained by substituting an equal volume of release medium [7].
Evaluation of solid SNEDDS
Pre-formulation evaluation of solid SNEDDS
Carr’s Index and Hausner’s ratio
These two measures the compressibility of powder. 10 g of powder was filled in a 50 mL graduated measuring cylinder. The initial volume was recorded. Then it was tapped 50 times in a graduated measuring cylinder [9]. The final volume was recorded. From the initial bulk volume (V0) and final tapped volume (Vt), Carr's Index and Hausner's ratio were determined as per Eqs. 1 and 2.
Angle of repose
The tilting box method was used to determine the Angle of repose. A fixed quantity of obtained Solid SNEDDS was weighed and placed within a box with a transparent side to observe the angle of the slide. Then the box was slowly tilted at a rate of approximately 3 degrees/second. And tilting was stopped when the Solid SNEDDS begin to slide in bulk, and the angle of tilt was measured.
Scanning electron microscopy
The surface topography of solid SNEDDS was studied using Scanning Electron Microscopy. Samples were produced by gently sprinkling solid SMEDDS powder on a double adhesive tape that was applied to a stub. The stubs were then coated with platinum in an argon atmosphere using a gold sputter module in a high vacuum evaporator. The samples were then randomly scanned and photographs with a higher magnification were taken for surface morphology. It produces the image by scanning with a beam of electrons. The sample was then scanned under Electron Microscope HITACHISU 1500 Japan, connected with a fine coat, JEOL JFC-1100E Ion sputter [10].
Post-formulation evaluations
Hardness test
Tablet hardness was measured using a Monsanto Hardness tester. The tablet was placed between the instrument’s jaw, and pressure was applied till the tablet got broken down. The hardness was measured in kg/cm2.
Disintegration test
The disintegration Test was performed as the per disintegration test procedure for uncoated tablets described in IP 2007. 0.1 N HCl was selected as media for the disintegration test maintained at 37 ± 2 °C.
Friability test
The Friability Test of tablets was performed according to official compendia. Ten tablets were weighed accurately before and after the friability test run. The difference in tablet weight was calculated in percentage.
Drug content
Ten tablets were selected and crushed in a clean mortar with a pestle and transferred into a 100-mL volumetric flask. The volumetric flask was filled with methanol up to mark and sonicated for 15 min. After sonication content of the volumetric flask was filtered using the Whatman filter paper and the absorbance was measured.
Stability study
Both liquid and solid SNEDDS underwent an accelerated stability assessment. The products were kept at 45 °C ± 2 °C and 75 ± 5% RH while stored in the same stability chamber. Over the course of one month, many physical and chemical parameters were assessed.
Results
Drug excipient compatibility study
FT-IR spectra of Ropinirole were recorded to check the compatibility of Ropinirole with excipients. Characteristic absorption bands in IR regions are shown in Fig. 3. Ropinirole showed characteristic peaks at 2932 cm−1 due to N–H stretching vibration, CH3 bending occurs at 1370, 1722 cm−1 due to carbonyl stretching vibration, and C=C stretching at 1370 cm−1 [11]. These peaks of Ropinirole were successfully obtained in combination with other solvents like Tween 20, Acrysol EL 135, and Capmul MCM C8. In the case of Tween 20, C=O stretching was found at 1735 cm−1, C=C stretching at 1456 cm−1, CH3 bending at 1354 cm−1, and NH bending at 2922 cm−1 which were all in the range as that of pure Ropinirole. Further, in Acrysol EL 135, C=O stretching was found at 1732 cm−1, C=C stretching at 1459 cm−1, CH3 bending at 1360 cm−1 and NH bending at 2866 cm−1. Similarly, for Capmul MCM C8, C=O stretching was found at 1735 cm−1, C=C stretching at 1455 cm−1, CH3 bending at 1376 cm−1 and NH bending at 2925 cm−1. These indicate that all functional groups that are present in Ropinirole are retained when Ropinirole is present in combinations with Tween 20, Acrysol EL 135, and Capmul MCM C 8. This further proves that there is no incompatibility between the selected solvent system and Ropinirole.
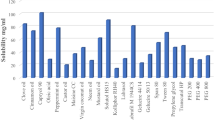
Solubility study of drugs in various oils, surfactants, and co-surfactants
Table 4 contains the findings of the solubilities of ropinirole in various oils, surfactants, and co-surfactants. Capmul MCM EP and Capmul MCM C8 exhibit the maximum solubility within the oil category. In the case of surfactants, Span 80 and Tween 20 show the highest solubility, whereas Transcutol HP and Acrysol EL 135 show the highest solubility in the case of co-surfactants.
Preliminary tests for choosing an excipient combination
See Table 5.
Construction of ternary phase diagram
Twenty-two SNEDDS batches were made and analyzed for % Transmittance and emulsification effectiveness. Based on Table 6, a separate grade was assigned to each batch. The batches that produce emulsion in a minute (grade A) and exhibit a greater than 90% transmittance were chosen. They create the nano-emulsification zone, as depicted in Fig. 4. The nano-emulsification zone is shown in the red area. The upper and lower levels of the solvent system are also identified by the Ternary plot, which is useful for the optimization process.
This Ternary plot also helps identify the solvent system's higher and lower level, which could be used later in the optimization process.
Optimization of formulation: Box–Behnken design
In preliminary trials, for the selection of an appropriate solvent system, self-emulsification efficiency was assessed by visual inspection and qualitative grading system. After selecting the suitable solvent system, for optimizing the concentration of the solvent system, the dependent variables selected were droplet size and rate of emulsification in seconds. From Table 7, it can be concluded that Droplet size decreases with an increase in the concentration of surfactant.
We can also say that droplet size change is inversely proportional to the concentration of surfactant. When the concentration of surfactant and co-surfactant was higher, the droplet size acquired its lowest value of nearby 39 nm approximately (ROP 26). Therefore, the proper combination of surfactant and co-surfactant can give SNEDDS with desirable droplet sizes.
Also, most of the formulations had an emulsification time of 1 min. Formulations ROP 24, 28, 35 have a high emulsification time of more than 60 s. These may be either due to the high concentration of oil or due to the low concentration of surfactant and co-surfactant (Figs. 5, 6, 7 and 8).
Check point batches
The checkpoint batches were prepared, to find the predictability of the model. Both predicted values and actual values of both responses were compared. Two checkpoint batches were prepared and studied for Droplet size and Rate of Emulsification. It was found that both the values are in accordance with each other as shown in Table 8.
A total of 30 solutions were provided by the model. From them, one solution was considered depending on the criteria of high oil content and less Rate of emulsification. The desirability was 0.861 (Table 9). The optimized solution contains 5 mg of the drug, 0.30 mL of oil (Capmul MCM EP), 2.40 mL of surfactant Tween 20, 2.10 mL of co-surfactant Acrysol EL 135.
Solidification of liquid SNEDDS
Estimation of optimum liquid: adsorbent ratio
Out of 3 adsorbents taken, Syloid 244 FP was selected as a suitable adsorbent based on the data as shown in Table 10. The 1:4 mixture of Syloid 244 FP with Liquid SNEDDS shows good flow properties compared to other adsorbents. Carr’s Index was found to be 12.7 (good), Hausner’s ratio was 1.154(good), and the Angle of Repose was 33.8 (good). Based on these findings, Syloid 244 FP was chosen as the adsorbent.
Ropinirole SNEDDS tablet preparation
See Table 11.
Evaluation of liquid SNEDDS
Evaluation of transparency
The transparency of the optimized batch was found to be 98%. The formulation's optical characteristics and droplet size improve with a higher transmittance percentage. It will promote quick disintegration [12].
Droplet size
The average particle size was found to be 96.71 nm (Fig. 9). Since it determines the rate and extent of drug release as well as absorption, the droplet size of the emulsion is a critical factor in self-emulsification effectiveness. Because of the presence of a co-surfactant, the SNEDDS has the smallest globule size compared to coarse emulsions [13]. Also, the Polydispersity Index (PDI) is less than 1. The obtained value is 0.37 which shows the formulation is stable.
SEM analysis
The image of SNEDDS (Fig. 10) indicates that the liquid SNEDDS is adsorbed on Syloid 244 FP as adsorbent and are not agglomerated and are present as fine separate particles.
Evaluation of solid SNEDDS tablet
See Table 12.
In vitro drug release studies
From the results, we can conclude that liquid and solid SNEDDS dramatically improves drug release (approximately 99%) compared to pure Ropinirole (30%). Also, a significant increase in drug release from solid and liquid SNEDDS was found after 25 min. It increases from 56 to 90% (approx.). But after 30 min no such significant increase was seen. This may have occurred because of the spontaneous formation of nano-emulsion.
SNEDDS tablet shows a little lower rate of drug release as compared to liquid SNEDDS. This may have occurred due to the highly effective adherence of adsorbent to the drug which results in resistance to drug release [14] (Fig. 11).
Stability study
See Table 13.
Discussion
In the present study, an attempt was made to formulate solid SNEDDS of Ropinirole, an Anti-Parkinsonian drug to bypass metabolism by the liver and enhance its solubility characteristics [15, 16]. The selection of a suitable solvent system of oil, surfactant, and co-surfactant was done using a solubility study. Two of each oil, surfactant, and co-surfactant with the highest drug solubilizing capacity were selected. Different combinations of them were prepared to find the most appropriate solvent system. Based on the study Capmul MCM EP as oil, Tween 20 as a surfactant, and Acrysol EL 135 as co-surfactant was selected. FTIR study was performed to find any incompatibilities present between Ropinirole, Capmul MCM EP, Tween 20, and Acrysol EL 135. Based on the Ternary plot a total of 22 batches were prepared to determine the nano-emulsification zone [17]. This Ternary plot also helps to identify the higher and lower level of the solvent system which was used later in the optimization process.
Optimization of the formulation was done using Box-Behnken Design. The concentration of oil (X1), surfactant (X2), and co-surfactant (X3) were selected as independent variables, and Droplet size (Y1), Rate of emulsification (Y2) were selected as dependent variables for optimization. Droplet size decreased with increasing the concentration of surfactant. When the concentration of surfactant and co-surfactant was higher, the droplet size acquired its least size of nearby 39 nm approximately (ROP 26). Therefore, the proper combination of surfactant and co-surfactant can give SNEDDS with desirable droplet size [18]. Also, most of the formulations had an emulsification time of 1 min. Checkpoint batches were prepared to evaluate the predictability of the optimization model. Optimized liquid SNEDDS was evaluated for droplet size (96.71 nm), percent transparency (98.90%), and in vitro drug release. The liquid SNEDDS was then converted into powder form by adsorption on Syloid 244 FP. The converted powder underwent SEM analysis.
It was then converted into a tablet by direct compression method by adding suitable excipients. Both pre-formulation and post-formulation parameters were studied. The final weight of each tablet formulated was 535 mg with all its excipients. In vitro drug release studies of pure drugs, liquid SNEDDS, and solid SNEDDS were compared. Solid SNEDDS dramatically improves drug release (approximately 99%) compared to pure Ropinirole (30%).
Conclusion
In the current study, Ropinirole SNEDDS was successfully formulated which could bypass the liver and thereby avoid extensive hepatic first-pass metabolism. Thus, it may help to overcome the bioavailability problem. The selection of the optimum concentration of oil, surfactant, and co-surfactant plays a crucial role in the success of emulsification. FTIR peak shows no incompatibility between Ropinirole and the selected solvent system. The formulation was optimized by Box-Behnken Design to get optimized formulation with the desired desirability. The droplet size in the optimized batch is 96.71 nm, and the rate of emulsification is 22 s. With Syloid 244FP as an adsorbent, the liquid SNEDDS was successfully transformed into solid SNEDDS.SEM images show that Liquid SNEDDS is adsorbed on the adsorbent, Syloid 244FP, and are present as fine separate particles without agglomerations compared to pure suspension. The in vitro investigation demonstrates enhanced Ropinirole drug release from liquid and solid SNEDDS. According to the stability analysis, the formula is stable at 45 °C ± 2 °C and 75% ± 5% RH. Finally, it can be said that the potential of Ropinirole SNEDDS for enhancing bioavailability issues is promising.
Availability of data and materials
The data generated and analyzed during this research work are included in this article, if any excess data are required, it will be available from the corresponding author on reasonable request.
Abbreviations
- API:
-
Active pharmaceutical ingredient
- RPM:
-
Rotations per minute
- SMEDDS:
-
Self-microemulsifying drug delivery system
- SNEDDS:
-
Self-nanoemulsifying drug delivery system
- FTIR:
-
Fourier transform infra-red spectroscopy
- EAQG:
-
Emulsion appearance qualification grading
- SEM:
-
Scanning electron microscopy
- NMT:
-
Not more than
- NLT:
-
Not less than
- IPA:
-
Isopropyl alcohol
- RH:
-
Relative humidity
- SD:
-
Standard deviation
- ROP:
-
Ropinirole
- DoE:
-
Design of expert
References
Nour AH (2018) Emulsion types, stability mechanisms, and rheology: a review. Int J Innov Res Sci Stud (IJIRSS) 1(1):14–21
Rajpoot K, Tekade M, Pandey V, Nagaraja S, Youngren-Ortiz SR, Tekade RK (2020) Self-micro emulsifying drug-delivery system: ongoing challenges and future ahead. In: Drug delivery systems. Academic Press, pp 393–454
Madagul JK, Parakh DR, Kumar RS, Abhang RR (2017) Formulation and evaluation of solid self-microemulsifying drug delivery system of chlorthalidone by spray drying technology. Drying Technol 35(12):1433–1449
Parakh DR, Patil MP, Sonawane SS, Kshirsagar SJ (2017) Application of factorial design approach in development and evaluation of self microemulsifying drug delivery system (SMEDDS) of mebendazole. J Pharm Investig 47(6):507–519
Madan JR, Patil K, Awasthi R, Dua K (2021) Formulation and evaluation of solid self-micro emulsifying drug delivery system for azilsartan medoxomil. Int J Polym Mater Polym Biomater 70(2):100–116
Cirri M, Roghi A, Valleri M, Mura P (2016) Development and characterization of fast-dissolving tablet formulations of glyburide based on solid self-microemulsifying systems. Eur J Pharm Biopharm 104:19–29
Seljak KB, Ilić IG, Gašperlin M, Pobirk AZ (2018) Self-microemulsifying tablets prepared by direct compression for improved resveratrol delivery. Int J Pharm 548(1):263–275
Seo YG, Kim DH, Ramasamy T, Kim JH, Marasini N, Oh YK, Kim DW, Kim JK, Yong CS, Kim JO, Choi HG (2013) Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int J Pharm 452(1–2):412–420
Borhade V, Nair H, Hegde D (2008) Design and evaluation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS PharmSciTech 9(1):13–21
Chandra SM, Dharan SS, Ajikumar A (2021) Formulation and evaluation of gastroretentive floating microballoons containing selected anti-ulcer drug. J Pharm Sci Res 13(1):49–63
Barcia E, Boeva L, García-García L, Slowing K, Fernández-Carballido A, Casanova Y, Negro S (2017) Nanotechnology-based drug delivery of ropinirole for Parkinson’s disease. Drug Deliv 24(1):1112–1123
Alhasani KF, Kazi M, Ibrahim MA, Shahba AA, Alanazi FK (2019) Self-nanoemulsifying ramipril tablets: a novel delivery system for the enhancement of drug dissolution and stability. Int J Nanomed 14:5435
Qi X, Qin J, Ma N, Chou X, Wu Z (2014) Solid self-microemulsifying dispersible tablets of celastrol: formulation development, charaterization and bioavailability evaluation. Int J Pharm 472(1–2):40–47
Prajapati ST, Joshi HA, Patel CN (2013) Preparation and characterization of self-microemulsifying drug delivery system of olmesartan medoxomil for bioavailability improvement. J Pharm 2013:1–9
Stocchi F, Hersh BP, Scott BL, Nausieda PA, Giorgi L, Ease-PD Monotherapy Study Investigators (2008) Ropinirole 24-hour prolonged release and ropinirole immediate release in early Parkinson's disease: a randomized, double-blind, non-inferiority crossover study. Curr Med Res Opin 24(10):2883–2895
Davie CA (2008) A review of Parkinson’s disease. Br Med Bull 86(1):109–127
Park SY, Jin CH, Goo YT, Chae BR, Yoon HY, Kim CH et al (2021) Supersaturable self-microemulsifying drug delivery system enhances dissolution and bioavailability of telmisartan. Pharm Dev Technol 26(1):60–68
Holm R, Jensen IHM, Sonnergaard J (2006) Optimization of self-microemulsifying drug delivery systems (SMEDDS) using a D-optimal design and the desirability function. Drug Dev Ind Pharm 32(9):1025–1032
Acknowledgements
We are thankful to Intas pharmaceutical Ltd. for kindly providing the API. The authors are thankful to Smt. S.M. Shah Pharmacy College for providing required facilities for carrying out the research work.
Funding
No funding was received for this research work.
Author information
Authors and Affiliations
Contributions
Author AM performed all the above research work and prepared the complete manuscript. Author KD and UV guided and monitored the research activities. Author HK and MJ contributed in final drafting of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declare no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Detholia, K., Mohandas, A., Varia, U. et al. Development and optimization of Ropinirole loaded self-nanoemulsifying tablets. Futur J Pharm Sci 9, 66 (2023). https://doi.org/10.1186/s43094-023-00516-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00516-x