Abstract
Product temperature during the primary drying step of freeze-drying is controlled by a set point chamber pressure and shelf temperature. However, recent computational modeling suggests a possible variation in local chamber pressure. The current work presents an experimental verification of the local chamber pressure gradients in a lab-scale freeze-dryer. Pressure differences between the center and the edges of a lab-scale freeze-dryer shelf were measured as a function of sublimation flux and clearance between the sublimation front and the shelf above. A modest 3-mTorr difference in pressure was observed as the sublimation flux was doubled from 0.5 to 1.0 kg·h−1·m−2 at a clearance of 2.6 cm. Further, at a constant sublimation flux of 1.0 kg·h−1·m−2, an 8-fold increase in the pressure drop was observed across the shelf as the clearance was decreased from 4 to 1.6 cm. Scale-up of the pressure variation from lab- to a manufacturing-scale freeze-dryer predicted an increased uniformity in drying rates across the batch for two frequently used pharmaceutical excipients (mannitol and sucrose at 5% w/w). However, at an atypical condition of shelf temperature of +10°C and chamber pressure of 50 mTorr, the product temperature in the center vials was calculated to be a degree higher than the edge vial for a low resistance product, thus reversing the typical edge and center vial behavior. Thus, the effect of local pressure variation is more significant at the manufacturing-scale than at a lab-scale and accounting for the contribution of variations in the local chamber pressures can improve success in scale-up.


Similar content being viewed by others
Notes
Pressure differences at clearances below 1.4 cm could not be measured due to equipment constraints.
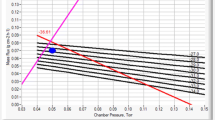
0.13 kg·h−1·m−2 × 2.7 mTorr/(kg·h−1·m−2) = 0.35 ≈ 0.5 mTorr. The value of 2.7 mTorr/(kg·h−1·m−2) taken from Fig. 2a.
Based on experience in our lab (21)
0.94 kg·h−1·m−2 × 2.7 mTorr (kg·h−1·m−2) = 2.5 mTorr. The value of 2.7 mTorr/(kg·h−1·m−2) taken from Fig. 2a.
A freeze-drying cycle is ideally optimized for process conditions to target a product temperature 2–3°C below the product’s critical temperature. An increase of 1.5°C in the product temperature due to the higher product resistance associated with a lower ice nucleation temperature can thus increase the risk of approaching or exceeding the critical temperature, causing loss of product cake structure.
The batch average nucleation temperature is based on compilation of 37 data points for ice nucleation in 20 cc vials in our lab (unpublished).
REFERENCES
Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–75.
Brülls M, Rasmuson A. Heat transfer in vial lyophilization. Int J Pharm. 2002;246(1):1–16.
Pikal MJ. Use of laboratory data in freeze drying process design: heat and mass transfer coefficients and the computer simulation of freeze drying. J Parenter Sci Technol. 1985;39(3):115–39.
Pikal MJ, Roy ML, Shah S. Mass and heat transfer in vial freeze-drying of pharmaceuticals: role of the vial. J Pharm Sci. 1984;73(9):1224–37.
Jansco G, Pupezin J, Van Hook WA. The vapor pressure of ice between 10–2 and − 10 2°C. J Phys Chem. 1970;74:2984–9.
Model-based monitoring of a non-uniform batch in a freeze-drying process. Proceedings of 18th European Symposium on Computer Aided Process Engineering–ESCAPE18 (edited by B. Braunschweig, X. Joulia); 2008.
Rasetto V, Marchisio DL, Fissore D, Barresi AA. On the use of a dual-scale model to improve understanding of a pharmaceutical freeze-drying process. J Pharm Sci. 2010;99(10):4337–50.
Zhang S, Liu J. Distribution of vapor pressure in the vacuum freeze-drying equipment. Math Probl Eng 2012;2012.
Varma N, Ganguly A, Sane P, Bogner R, Pikal M, Alexeenko A. Spatial variation of pressure in lyophilization product chamber part 1: computational modeling. Manuscript submitted to AAPS PharmSciTech.
Schneid SC, Gieseler H, Kessler WJ, Pikal MJ. Non-invasive product temperature determination during primary drying using tunable diode laser absorption spectroscopy. J Pharm Sci. 2009;98(9):3406–18.
Milton N, Pikal MJ, Roy ML, Nail SL. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. PDA J Pharm Sci Technol. 1997;51(1):7–16.
Patel SM, Chaudhuri S, Pikal MJ. Choked flow and importance of Mach I in freeze-drying process design. Chem Eng Sci. 2010;65(21):5716–27.
Konstantinidis AK, Kuu W, Otten L, Nail SL, Sever RR. Controlled nucleation in freeze-drying: effects on pore size in the dried product layer, mass transfer resistance, and primary drying rate. J Pharm Sci. 2011;100(8):3453–70.
Searles JA, Carpenter JF, Randolph TW. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J Pharm Sci. 2001;90(7):860–71.
Pikal MJ. Freeze drying. Encyclopedia of pharmaceutical technology. Marcel Dekker, New York 2002;1299:1326.
Liao X, Krishnamurthy R, Suryanarayanan R. Influence of processing conditions on the physical state of mannitol—implications in freeze-drying. Pharm Res. 2007;24(2):370–6.
Rambhatla S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, I: atypical radiation and the edge vial effect. AAPS PharmSciTech. 2003;4(2):111–20.
Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–63.
Kochs M, Körber C, Heschel I, Nunner B. The influence of the freezing process on vapour transport during sublimation in vacuum-freeze-drying of macroscopic samples. Int J Heat Mass Transf. 1993;36(7):1727–38.
Rambhatla S, Ramot R, Bhugra C, Pikal MJ. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling. Aaps Pharmscitech. 2004;5(4):54–62.
Sane P, Pikal M, Nail S, Bogner R. Measurement of heat transfer to vials on a freeze-dryer shelf during primary drying. Manuscript in preparation.
Kobayashi M, Harashima K, Ariyama H, Yao A. Inter-vial variance of the sublimation rate in shelf freeze-dryer. Trans Jpn Soc Refrigerating Air Conditioning Eng. 2011;10:135–44.
Pikal M, Rambhatla S, Ramot R. The impact of the freezing stage in lyophilization: effects of the ice nucleation temperature on process design and product quality. Am Pharm Rev. 2002;5:48–53.
Roy ML, Pikal MJ. Process control in freeze drying: determination of the end point of sublimation drying by an electronic moisture sensor. PDA J Pharm Sci Technol. 1989;43(2):60–6.
Sane P, Agarabi C, Pikal M, Bogner R. Quantifying the variation in product temperature and drying time within a lyophilized batch during primary drying. Manuscript in preparation.
ACKNOWLEDGMENTS
This work was done in collaboration with Cyrus Agarabi, Mansoor Khan, and Rakhi Shah from FDA and was funded by FDA U01-PU002-2012 through the National Institute for Pharmaceutical Technology and Education (NIPTE).
Author information
Authors and Affiliations
Corresponding author
APPENDICES
APPENDICES
I. Determination of the zero-point offset value for ΔP c→e measurements:
Unexpectedly, at conditions expected to produce no pressure variation, the differential CM displayed a non-zero value. For example, when the drying chamber door was closed, but before cooling the shelf to produce the ice slab (Protocol 1 in Table VII), there was no vapor flow from sublimation to produce a pressure drop; the differential CM was expected to produce a value close to zero for ΔP c→e, but typically did not. Additionally, at the end of collecting ΔP c→e at each chamber pressure, the shelf with the ice slab was lowered to achieve maximum distance (i.e., 14 cm) from the false upper shelf (Protocol 2 in Table VII), where the pressure difference, ΔP c→e, at this gap distance was expected to be negligible (Fig. 2b).
However, neither Protocol in Table VII produced ΔP c→e readings that could be used as zero-point offset values to obtain meaningful data. Instead, values of ΔP c→e ranged from 0.4 to 45.0 mTorr, when a zero value was expected. There was no trend in the “zero point” ΔP c→e measurements with increasing chamber pressure (Table VIII). However, when the zero-point differential pressure readings were listed in order of collection date, there was an obvious increase in zero point as a function of time (Table IX). The drift over time is most likely due to small permanent deflections of the diaphragm in the differential CM that result in hysteresis. The analysis of ΔP c→e in this report assumed that only the zero-point offset is affected and not the sensitivity of the diaphragm deflections to pressure changes.
The CFD studies of Varma et al demonstrated a linear dependence of pressure difference on sublimation flux. If the raw ΔP c→e data were extrapolated to a sublimation flux of 0 kg∙h−1∙m−2, the intercept should provide the most reliable value for the zero-point offset for pressure difference. Hence, as an alternative to either one of the directly measured values for zero-point offset (i.e., Protocol 1 or 2), a third value (Protocol 3 in Table IX) was derived from extrapolation of the data for ΔP c→e as a function of sublimation rate (Fig. 2a) using zero point as determined from protocol 2 to zero sublimation rate. Clearly, at zero sublimation rate, the pressure difference must be zero, and as indicated above, the expectation is that the pressure difference should be essentially linear in sublimation rate.
II. Effect of variation in nucleation temperature on the resistance of the dry layer to water vapor transport
In a freeze-drying cycle, as the shelf temperature is lowered, not all vials contain ice at the equilibrium freezing point of the solution. In fact, normally none do! The product in the vial can exist in the liquid state well below the equilibrium freezing point (i.e., −10 to −20°C). The degree of supercooling prior to ice nucleation during ramp freezing largely determines the size of the ice crystals (24). When ice leaves the frozen product by sublimation during primary drying, the porous structure of the solute it leaves behind is essentially a template of the ice removed. Any variation in degree of supercooling leads to a variation in the porosity of the freeze-dried solute and the resistance offered by the dried product to vapor flow during primary drying. Higher degrees of supercooling are associated with smaller ice crystals, leading to the formation of small pores in the dried layer and greater resistance to water vapor transport.
Previous experience in our lab with 20 cc vials in a lab freeze-dryer (Lyostar 2) has shown a range of ice nucleation temperatures from −6 to −20°C, with 80% of vials undergoing ice nucleation between −8 and −17°C (25). This range of ice nucleation temperatures leads to a range in dried layer resistance for vials within a batch, which was calculated as described next.
The dry layer resistance of both sucrose and mannitol at a concentration of 5% w/w was determined using Manometric Temperature Measurement (MTM) (11) at ice nucleation temperatures of −5 and −10°C using the Praxair ControLyo™ Nucleation. The product resistance as a function of the dry layer thickness was fitted by the empirical relationship:
where R p is the measured product resistance to the water vapor leaving the ice surface through the dried cake of thickness, l (3). The parameters, R o, A 1, and A 2, are used to fit the shape of the R p versus l curve. For both sucrose and mannitol, the resistance was related to nucleation temperature by an ice nucleation temperature-dependent factor, F Tnuc, specific for that material as described below. The values of R o, A 1, and A 2 were also material specific.
The values of R o, A 1, and A 2 were determined for each material such that multiplication of R p (Eq. 4) by a factor, F T,nuc, described the resistance curves at both −5 and −10°C, reasonably well. The temperature-dependent factor allowed for an increase in resistance as ice nucleation temperature decreased, while maintaining the general shape of the resistance versus dry layer thickness curve. In determining the values of F T,nuc, several assumptions were made.
-
i.
The change in resistance between −5 and −10°C is linear and can be extrapolated to lower ice nucleation temperatures (This is perhaps the weakest assumption).
-
ii.
The cake resistance, R p, and the change in resistance with nucleation temperature, \( \frac{d{R}_{\mathrm{p}}}{d{T}_{\mathrm{nuc}}} \), are about 25% lower for products in which ice nucleation was initiated at a single temperature as compared to product in vials where ice nucleated spontaneously, but at the same temperature.
-
iii.
The average nucleation temperature in a batch without nucleation control is −15°C.Footnote 6
Based on these assumptions, the average product resistance values for each material obtained from controlled nucleation runs were multiplied by factors such that the resistance linearly extrapolated to a nucleation temperature of −15°C equaled that found during uncontrolled nucleation.
The measured average cake resistance values for 5% w/w sucrose at ice nucleation temperatures of −5 and −10°C were 1.70 and 2.47 cm2∙h∙Torr∙g−1, respectively, and the average resistance for the same material without ice nucleation control was 3.91 cm2∙h∙Torr∙g−1 (Table X). When the value of the experimentally measured resistance of the 5% w/w sucrose cakes frozen using nucleation control is extrapolated to −15°C and multiplied by 1.25 (according to assumption ii above), it agrees well with resistance in the absence of nucleation control. The same factor 1.25 works well for both sucrose and mannitol.
Using the value for the change in resistance per degree \( \left(\frac{d{R}_{\mathrm{p}}}{d{T}_{\mathrm{nuc}}}\right) \), the average product resistance for a low ice nucleation temperature of −17°C and a high ice nucleation temperature of −8°C were estimated. In the case of mannitol, the average product resistance varies from 6.15 cm2∙h∙Torr∙g−1 when ice nucleation occurs at −8°C to 8.13 cm2∙h∙Torr∙g−1 when ice nucleation occurs at −17°C. Sucrose at 5% has a lower dry layer resistance (i.e., 2.70 cm2∙h∙Torr∙g−1 when ice nucleation occurs at −8°C and 4.42 cm2∙h∙Torr∙g−1 when ice nucleation occurs at −17°C).
Rights and permissions
About this article
Cite this article
Sane, P., Varma, N., Ganguly, A. et al. Spatial Variation of Pressure in the Lyophilization Product Chamber Part 2: Experimental Measurements and Implications for Scale-up and Batch Uniformity. AAPS PharmSciTech 18, 369–380 (2017). https://doi.org/10.1208/s12249-016-0502-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0502-6