Abstract
Purpose
To study the effect of processing conditions on the physical state of mannitol during various stages of the lyophilization cycle of a protein formulation.
Materials and Methods
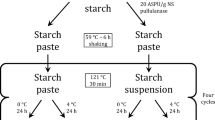
Mannitol and trehalose were used as the bulking agent and lyoprotectant, respectively. The physical state of mannitol during various stages of freeze-drying cycle, in the absence and presence of a model protein, was characterized using low temperature X-ray powder diffractometry (XRD) and differential scanning calorimetry (DSC).
Results
Mannitol did not crystallize even when the solution for lyophilization was cooled to −45°C at a slow cooling rate of 1°C/min. Annealing facilitated mannitol crystallization, and in the absence of the protein, a mixture of δ-mannitol and mannitol hemihydrate was obtained at both low (−18°C) and high (−8°C) annealing temperatures. However, in the presence of protein, the high annealing temperature promoted δ-mannitol crystallization and inhibited formation of mannitol hemihydrate, while the low annealing temperature facilitated the formation of mannitol hemihydrate. Interestingly, the hemihydrate in the frozen solution was retained in the final lyophile, even when the primary and secondary drying temperatures were as high as −5 and 65°C, respectively.
Conclusions
The presence of protein as well as the processing conditions (annealing temperature and time, primary and secondary drying temperatures) influenced the physical form of mannitol in the final lyophile. The protein promoted formation of δ-mannitol while inhibiting the formation of mannitol hemihydrate. Since the physical form of mannitol was greatly influenced by the presence of protein, it will be prudent to conduct the preliminary lyophilization cycle development studies in the presence of the protein. If mannitol hemihydrate is formed during annealing, its dehydration may require high secondary drying temperature.









Similar content being viewed by others
References
D. Q. Wang, J. M. Hey, and S. L. Nail. Effect of collapse on the stability of freeze-dried recombinant factor VIII and a-amylase. J. Pharm. Sci. 93:1253–1263 (2004).
R. E. Johnson, C. F. Kirchhoff, and H. T. Gaud. Mannitol–sucrose mixtures—versatile formulations for protein lyophilization. J. Pharm. Sci. 91:914–922 (2002).
K. Izutsu, S. Yoshioka, and T. Terao. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm. Res. 10:1232–1237 (1993).
A. I. Kim, M. J. Akers, and S. L. Nail. The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J. Pharm. Sci. 87: 931–935 (1998).
S. Torrado and S. Torrado. Characterization of physical state of mannitol after freeze-drying: effect of acetylsalicylic acid as a second crystalline cosolute. Chem. Pharm. Bull. 50:567–570 (2002).
R. K. Cavatur, N. M. Vemuri, A. Pyne, Z. Chrzan, D. Toledo-Velasquez, and R. Suryanarayanan. Crystallization behavior of mannitol in frozen aqueous solutions. Pharm. Res. 19:894–900 (2002).
A. J. Cannon and E. H. Trappler. The influence of lyophilization on the polymorphic behavior of mannitol. PDA J. Pharm. Sci. Technol. 54:13–22 (2000).
R. Haikala, R. Eerola, V. P. Tanninen, and J. Yliruusi. Polymorphic changes of mannitol during freeze-drying: effect of surface-active agents. PDA J. Pharm. Sci. Technol. 51:96–101 (1997).
L. Yu, N. Milton, E. G. Groleau, D. S. Mishra, and R. E. Vansickle. Existence of a mannitol hydrate during freeze-drying and practical implications. J. Pharm. Sci. 88:196–198 (1999).
A. Pyne, K. Chatterjee, and R. Suryanarayanan. Solute crystallization in mannitol–glycine systems—implications on protein stabilization in freeze-dried formulations. J. Pharm. Sci. 92:2272–2283 (2003).
C. Telang, L. Yu, and R. Suryanarayanan. Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride. Pharm. Res. 20:660–667 (2003).
X. Liao, R. Krishnamurthy, and R. Suryanarayanan. Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol-implications in freeze-drying. Pharm. Res. 22:1978–1985 (2005).
E. Leikina, M. V. Mertts, N. Kuznetsova, and S. Leikin. Type I collagen is thermally unstable at body temperature. Proc. Natl. Acad. Sci. U. S. A. 99:1314–1318 (2002).
J. C. Bischof and X. He. Thermal stability of proteins. Ann. N.Y. Acad Sci. 1066:12–33 (2005).
A. Pyne, K. Chatterjee, and R. Suryanarayanan. Crystalline to amorphous transition of disodium hydrogen phosphate during primary drying. Pharm. Res. 20:802–803 (2003).
S. Chongprasert, U. J. Griesser, A. T. Bottorff, N. A. Williams, S. R. Byrn, and S. L. Nail. Effects of freeze-dry processing conditions on the crystallization of pentamidine isethionate. J. Pharm. Sci. 87:1155–1160. (1998).
A. Pyne and R. Suryanarayanan. Phase transitions of glycine in frozen aqueous solutions and during freeze-drying. Pharm. Res. 18:1448–1454 (2001).
W. Wang, Y. Ou, and Y. Shi. AlbuBNP, a recombinant B-type natriuretic peptide and human serum albumin fusion hormone, as a long-term therapy of congestive heart failure. Pharm. Res. 21:2105–2111 (2004).
B. L. Osborn, L. Sekut, M. Corcoran, C. Poortman, B. Sturm, G. Chen, D. Mather, H. L. Lin, and T. J. Parry. Albutropin: a growth hormone–albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur. J. Pharmacol. 456:149–158 (2002).
B. L. Osborn, H. S. Olsen, B. Nardelli, J. H. Murray, J. X. H. Zhou, A. Garcia, G. Moody, L. S. Zaritskaya, and C. Sung. Pharmacokinetic and pharmacodynamic studies of a human serum albumin–interferon—a fusion protein in cynomolgus monkeys. J. Pharmacol. Exp. Ther. 303:540–548 (2002).
A. Pyne, R. Surana, and R. Suryanarayanan. Crystallization of mannitol below Tg′ during freeze-drying in binary and ternary aqueous systems. Pharm. Res. 19:901–908 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, X., Krishnamurthy, R. & Suryanarayanan, R. Influence of Processing Conditions on the Physical State of Mannitol—Implications in Freeze-Drying. Pharm Res 24, 370–376 (2007). https://doi.org/10.1007/s11095-006-9158-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9158-3