Abstract
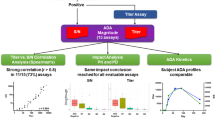
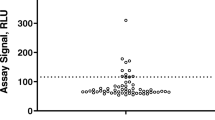
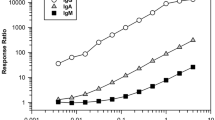
Historically, the biopharmaceutical industry has used titer to characterize the magnitude of an anti-drug antibody (ADA) response. While reporting levels of antibodies in terms of titer is generally understood and accepted by regulatory and medical communities, titer values are inherently variable given the multiple serial dilutions and reporting a value either directly before or interpolated at the assay cut point on the lower plateau of the assay curve range. Using S/N is an appealing alternative approach to titer as it simplifies analysis with less dilutions, significantly reducing testing, time, and resources and provides a more precise value potentially differentiating low-level ADA responses. Current bridging electrochemiluminescence (ECL) ADA assays using Meso Scale Discovery (MSD) platform are also significantly more sensitive and drug tolerant with wider assay ranges compared to historic ELISA platforms; therefore, ADA response based on S/N may help differentiate and identify those ADA samples that are more likely to be clinically relevant. Bococizumab is a humanized monoclonal antibody targeting proprotein convertase subtilisin-kexin type 9 (PCSK9), which reduces plasma levels of low-density lipoprotein (LDL) cholesterol. Bococizumab was discontinued during Phase 3 clinical development based in part on the high rate of ADA and wide variation in LDL cholesterol responses among patients. The impact of anti-bococizumab antibodies on pharmacokinetic (PK) and pharmacodynamic (PD) endpoints was originally assessed using titer. Retrospective analysis of anti-bococizumab ADA responses using S/N ratios illustrates that S/N is an acceptable alternative to titer for characterizing the magnitude of ADA response and interpretation of clinically relevant ADA.
Graphical Abstract





© 2017 Massachusetts Medical Society. Reprinted with permission (6)

© 2017 Massachusetts Medical Society. Reprinted with permission (6)

© 2017 Massachusetts Medical Society. Reprinted with permission (6)


Similar content being viewed by others
References
FDA. Immunogenicity testing of therapeutic protein products —developing and validating assays for anti-drug antibody detection. Guidance for industry. U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). 2019. Available from: https://www.fda.gov/media/119788/download.
Committee for Medicinal Products for Human Use (CHMP). Guideline on immunogenicity assessment of therapeutic proteins. 2017. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf.
Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658–73. https://doi.org/10.1208/s12248-014-9599-2.
Manning MS, Hassanein M, Partridge MA, Jawa V, Mora J, Ryman J, et al. Comparison of titer and signal to noise (S/N) for determination of anti-drug antibody magnitude using clinical data from an Industry Consortium. AAPS J. 2022;24:81. https://doi.org/10.1208/s12248-022-00728-8.
Manning MS, Kroenke MA, Lee SA, Harrison SE, Hoofring SA, Mytych DT, Jawa V. Assay signal as an alternative to titer for assessment of magnitude of an antidrug antibody response. Bioanalysis. 2017;9(23):1849–58. https://doi.org/10.4155/bio-2017-0185.
Ridker PM, Tardif J-C, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376:1517–26. https://doi.org/10.1056/NEJMoa1614062.
Ridker PM, Amarenco P, Brunell R, Glynn RJ, Jukema JW, Kastelein JP, et al. Evaluating bococizumab, a monoclonal antibody to PCSK9, on lipid levels and clinical events in broad patient groups with and without prior cardiovascular events: rationale and design of the Studies of PCSK9 Inhibition and the Reduction of Vascular Events (SPIRE) Lipid Lowering and SPIRE Cardiovascular Outcomes Trials. Am Heart J. 2016;178:135–44. https://doi.org/10.1016/j.ahj.2016.05.010.
Guidance for Industry, Bioanalytical Method Validation. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). 2001 and 2018. Available from: https://www.fda.gov/media/70858/download.
Committee for Medicinal Products for Human Use (CHMP). Guideline on immunogenicity assessment of therapeutic proteins. 2007. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-biotechnology-derived-therapeutic-proteins-first-version_en.pdf.
FDA: Draft Guidance for Industry: Assay Development and validation for Immunogenicity Testing of Therapeutic Protein Products. 2009 and 2016. https://www.fda.gov/media/77796/download.
Ridker PM, Tardif J-C, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. Supplementary material. N Engl J Med. 2017;376:1517–5126. https://doi.org/10.1056/NEJMoa1614062.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18(6):499–502. https://pubmed.ncbi.nlm.nih.gov/4337382.
Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658–73. https://doi.org/10.1208/s12248-014-9599-2.
USP. United States Pharmacopoiea General Chapter 1106: immunogenicity assays – design and validation of immunoassays to detect anti-drug antibodies. 2014. p. 909–22
Myler H, Gorovits B, Phillips K, Devanarayan V, Clements-Egan A, Gunn GR, et al. Report on the AAPS Immunogenicity Guidance Forum. AAPS J. 2019;21:55. https://doi.org/10.1208/s12248-019-0328-8.
Song S, Yang L, Trepicchio WL, Wyant T. Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. J Immunol Res. 2016. https://doi.org/10.1155/2016/3072586.
Acknowledgements
The authors would like to thank Naren Surampalli for supporting all the LDL cholesterol, bococizumab, and PCSK9 data programming using S/N. The authors would also like to thank Meghana Deshpande and Katrina Olson for their detailed quality data review.
Funding
This work was exclusively supported by Pfizer, Inc. SPIRE ClinicalTrials.gov numbers: SPIRE-HR (NCT01968954), SPIRE-LDL (NCT01968967), SPIRE-FH (NCT01968980), and SPIRE-LL (NCT02100514).
Author information
Authors and Affiliations
Contributions
All authors were involved in contributing and interpreting data, reviewed the final version of the manuscript, and have met the criteria for authorship as established by the ICMJE.
Corresponding author
Ethics declarations
Conflict of Interest
This study was funded by Pfizer, Inc. All the authors listed are employees of the Pfizer, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCush, F., Wang, E., Yunis, C. et al. Anti-drug Antibody Magnitude and Clinical Relevance Using Signal to Noise (S/N): Bococizumab Case Study. AAPS J 25, 85 (2023). https://doi.org/10.1208/s12248-023-00846-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00846-x