Abstract
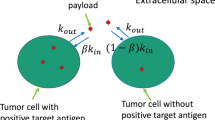
Antibody-drug conjugates (ADCs) are cancer drugs composed of a humanized antibody linked to a cytotoxic payload, allowing preferential release of payload in cancer cells expressing the antibody-targeted antigen. Here, a systems pharmacology model is used to simulate ADC transport from blood to tumor tissue and ADC uptake by tumor cells. The model includes effects of spatial gradients in drug concentration in a three-dimensional network of tumor blood vessels with realistic geometry and accounts for diffusion of ADC in the tumor extracellular space, binding to antigen, internalization, intracellular processing, and payload efflux from cells. Cells that process an internalized ADC-antigen complex may release payload that can be taken up by other “bystander” cells. Such bystander effects are included in the model. The model is used to simulate conditions in previous experiments, showing good agreement with experimental results. Simulations are used to analyze the relationship of bystander effects to payload properties and single-dose administrations. The model indicates that exposure of payload to cells distant from vessels is sensitive to the free payload diffusivity in the extracellular space. When antigen expression is heterogeneous, the model indicates that the amount of payload accumulating in non-antigen-expressing cells increases linearly with dose but depends only weakly on the percentage of antigen-expressing cells. The model provides an integrated mechanistic framework for understanding the effects of spatial gradients on drug distribution using ADCs and for designing ADCs to achieve more effective payload distribution in solid tumors, thereby increasing the therapeutic index of the ADC.







Similar content being viewed by others
References
Chari RVJ, Miller ML, Widdison WC. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed. 2014;53(15):3796–827. https://doi.org/10.1002/anie.201307628.
Alley SC, Zhang X, Okeley NM, Anderson M, Law C-L, Senter PD, et al. The pharmacologic basis for antibody-auristatin conjugate activity. J Pharmacol Exp Ther. 2009;330(3):932–8. https://doi.org/10.1124/jpet.109.155549.
Sanderson RJ, Hering MA, James SF, Sun MMC, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11:843–52.
Hamblett KJ, Senter PD, Chace DF, Sun MMC, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. https://doi.org/10.1158/1078-0432.ccr-04-0789.
Bakhtiar R. Antibody drug conjugates. Biotechnol Lett. 2016;38(10):1655–64. https://doi.org/10.1007/s10529-016-2160-x.
Liu C, Tadayoni BM, Bourret LA, Mattocks KM, Derr SM, Widdison WC, et al. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc Natl Acad Sci. 1996;93(16):8618–23.
Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–21. https://doi.org/10.1158/0008-5472.can-05-3973.
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16(3):888–97. https://doi.org/10.1158/1078-0432.ccr-09-2069.
Li F, Emmerton KK, Jonas M, Zhang X, Miyamoto JB, Setter JR, et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 2016;76(9):2710–9. https://doi.org/10.1158/0008-5472.can-15-1795.
Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody–drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13(6):1537–48. https://doi.org/10.1158/1535-7163.mct-13-0926.
Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66(8):4426–33. https://doi.org/10.1158/0008-5472.can-05-4489.
Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12(11):958–62. https://doi.org/10.1038/nmat3792.
Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52(19):5144–53.
Widdison WC, Ponte JF, Coccia JA, Lanieri L, Setiady Y, Dong L, et al. Development of anilino-maytansinoid ADCs that efficiently release cytotoxic metabolites in cancer cells and induce high levels of bystander killing. Bioconjug Chem. 2015;26(11):2261–78. https://doi.org/10.1021/acs.bioconjchem.5b00430.
Cormier JN, Hijazi YM, Abati A, Fetsch P, Bettinotti M, Steinberg SM, et al. Heterogeneous expression of melanoma-associated antigens and HLA-A2 in metastatic melanoma in vivo. Int J Cancer. 1998;75(4):517–24. https://doi.org/10.1002/(SICI)1097-0215(19980209)75:4<517::AID-IJC5>3.0.CO;2-W.
Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99(1):100–9.
Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59. https://doi.org/10.1007/s10928-012-9276-y.
Singh AP, Shah DK. Measurement and mathematical characterization of cell-level pharmacokinetics of antibody-drug conjugates: a case study with trastuzumab-vc-MMAE. Drug Metab Dispos. 2017;45:1120–32.
Singh AP, Shin YG, Shah DK. Application of pharmacokinetic-pharmacodynamic modeling and simulation for antibody-drug conjugate development. Pharm Res. 2015;32(11):3508–25. https://doi.org/10.1007/s11095-015-1626-1.
Cilliers C, Guo H, Liao J, Christodolu N, Thurber GM. Multiscale modeling of antibody-drug conjugates: connecting tissue and cellular distribution to whole animal pharmacokinetics and potential implications for efficacy. AAPS J. 2016;18(5):1117–30. https://doi.org/10.1208/s12248-016-9940-z.
Vasalou C, Helmlinger G, Gomes B. A mechanistic tumor penetration model to guide antibody drug conjugate design. PLoS One. 2015;10(3):e0118977. https://doi.org/10.1371/journal.pone.0118977.
Khera E, Cilliers C, Bhatnagar S, Thurber GM. Computational transport analysis of antibody-drug conjugate bystander effects and payload tumoral distribution: implications for therapy. Mol Syst Des Eng. 2018;3(1):73–88. https://doi.org/10.1039/C7ME00093F.
Secomb TW, Hsu R, Dewhirst MW, Klitzman B, Gross JF. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys. 1993;25(3):481–9. https://doi.org/10.1016/0360-3016(93)90070-C.
Hicks KO, Pruijn FB, Secomb TW, Hay MP, Hsu R, Brown JM, et al. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer Inst. 2006;98(16):1118–28. https://doi.org/10.1093/jnci/djj306.
Secomb TW. A Green’s function method for simulation of time-dependent solute transport and reaction in realistic microvascular geometries. Math Med Biol. 2016;33(4):475–94. https://doi.org/10.1093/imammb/dqv031.
Rhoden JJ, Wittrup KD. Dose dependence of intratumoral perivascular distribution of monoclonal antibodies. J Pharm Sci. 2012;101(2):860–7. https://doi.org/10.1002/jps.22801.
Secomb TW, Hsu R, Braun RD, Ross JR, Gross JF, Dewhirst MW. Theoretical simulation of oxygen transport to tumors by three-dimensional networks of microvessels. In: Hudetz AG, Bruley DF, editors. Oxygen Transport to Tissue XX. Boston: Springer US; 1998. p. 629–34.
Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–402. https://doi.org/10.1182/blood-2014-09-598763.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. https://doi.org/10.1111/cas.12966.
Silence K, Dreier T, Moshir M, Ulrichts P, Gabriels SME, Saunders M, et al. ARGX-110, a highly potent antibody targeting CD70, eliminates tumors via both enhanced ADCC and immune checkpoint blockade. mAbs. 2014;6(2):523–32. https://doi.org/10.4161/mabs.27398.
Wang R, Li L, Zhang S, Li Y, Wang X, Miao Q, et al. A novel enediyne-integrated antibody–drug conjugate shows promising antitumor efficacy against CD30+ lymphomas. Mol Oncol. 2018;12(3):339–55. https://doi.org/10.1002/1878-0261.12166.
Schmidt MM, Thurber GM, Wittrup KD. Kinetics of anti-carcinoembryonic antigen antibody internalization: effects of affinity, bivalency, and stability. Cancer Immunol Immunother. 2008;57(12):1879–90. https://doi.org/10.1007/s00262-008-0518-1.
Adam PJ, Terrett JA, Steers G, Stockwin L, Loader JA, Fletcher GC, et al. CD70 (TNFSF7) is expressed at high prevalence in renal cell carcinomas and is rapidly internalised on antibody binding. Br J Cancer. 2006;95:298–306. https://doi.org/10.1038/sj.bjc.6603222.
Sutherland MSK, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–7. https://doi.org/10.1074/jbc.M510026200.
Maass KF, Kulkarni C, Betts AM, Wittrup KD. Determination of cellular processing rates for a trastuzumab-maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J. 2016;18(3):635–46. https://doi.org/10.1208/s12248-016-9892-3.
El-Kareh AW, Secomb TW. Two-mechanism peak concentration model for cellular pharmacodynamics of doxorubicin. Neoplasia. 2005;7(7):705–13. https://doi.org/10.1593/neo.05118.
El-Kareh AW, Labes RE, Secomb TW. Cell cycle checkpoint models for cellular pharmacology of paclitaxel and platinum drugs. AAPS J. 2008;10(1):15–34. https://doi.org/10.1208/s12248-007-9003-6.
Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–37. https://doi.org/10.1038/nrd.2016.268.
Funding
This work was supported by the NIH National Heart, Lung and Blood Institute, grant HL034555.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 316 kb)
Rights and permissions
About this article
Cite this article
Burton, J.K., Bottino, D. & Secomb, T.W. A Systems Pharmacology Model for Drug Delivery to Solid Tumors by Antibody-Drug Conjugates: Implications for Bystander Effects. AAPS J 22, 12 (2020). https://doi.org/10.1208/s12248-019-0390-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0390-2