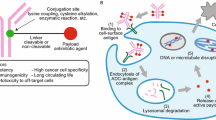
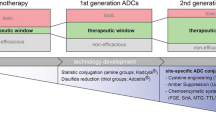
Abstract
Antibody drug conjugates (ADCs) have emerged as a viable option in targeted delivery of highly potent cytotoxic drugs in treatment of solid tumors. At the time of writing, only two ADCs have received regulatory approval with >40 others in clinical development. The first generation ADCs suffered from a lack of specificity in amino acid site-conjugations, yielding statistically heterogeneous stoichiometric ratios of drug molecules per antibody molecule. For the second generation ADCs, however, site-specific amino acid conjugation using enzymatic ligation, introduction of unnatural amino acids, and site-specific protein engineering hold promise to alleviate some of the current technical limitations. The rapid progress in technology platforms and antibody engineering has introduced novel linkers, site-specific conjugation chemistry, and new payload candidates that could possibly be exploited in the context of ADCs. A search using the Clinical Trial Database registry (www.clinicaltrials.gov), using the keyword ‘antibody drug conjugate’, yielded ~270 hits. The main focus of this article is to present a brief overview of the recent developments and current challenges related to ADC development.


Similar content being viewed by others
References
Adem YT, Schwarz KA, Duenas E, Patapoff TW, Galush WJ, Esue O (2014) Auristatin antibody drug conjugate physical instability and the role of drug payload. Bioconj Chem 25:656–664
AlDeghaither D, Smaglo BG, Weiner LM (2015) Beyond peptides and mAbs—current status and future perspectives for biotherapeutics with novel constructs. J Clin Pharmacol 55:S4–S20
Bae YH, Park K (2011) Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 153:198–205
Bakhtiar R (2012) Therapeutic recombinant monoclonal antibodies. J Chem Educ 89:1537–1542
Barok M, Joensuu H, Isola J (2014) Trastuzumab amtansine: mechanisms of action and drug resistance. Breast Cancer Res 16:209
Beckley NS, Lassareschi KP, Chih H-W, Sharma VK, Flores HL (2013) Investigation into temperature-induced aggregation of an antibody drug conjugate. Bioconj Chem 24:1674–1683
Behrens CR, Liu B (2014) Methods for site-specific drug conjugation to antibodies. MAbs 6:1–8
Blencowe CA, Russell AT, Greco F, Hayes W, Thornthwaite DW (2011) Self-immolative linkers in polymeric delivery systems. Polym Chem 2:773–790
Chari RVJ (2008) Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res 41(1):98–107
Chari RVJ, Miller ML, Widdison WC (2014) Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed 53:3796–3827
Chiche J, Brahimi-Horn MC, Pouyssegur J (2010) Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 14:771–794
Choi I-K, Strauss R, Richter M, Yun C-O, Lieber A (2013) Strategies to increase drug penetration in solid tumors. Front Oncol 3(Article 193):1–18
Correia IR (2010) Stability of IgG isotypes in serum. MAbs 2:221–231
de Goeij BECG, Lambert JM (2016) New develoipments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol 40:14–23
Deonarain MP, Yahioglu G, Stamati I, Marklew J (2015) Emerging formats for next-generation antibody drug conjugates. Expert Opin Drug Discov 10:463–481
Dhillon S (2014) Trastuzumab emtansine: a review of its use in patients with HER2-positive advanced breast cancer previously treated with trastuzumab-based therapy. Drugs 74:675–686
Diamantis N, Banerji U (2016) Antibody-drug conjugates-an emerging class of cancer treatment. Br J Cancer 114:362–367
Donaghy H (2016) Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs, In Press (DOI:10.1080/19420862.2016.1156829)
Dosio F, Stella B, Cerioni S, Gastaldi D, Arpicco S (2014) Advances in anticancer antibody-drug conjugates and immunotoxins. Recent Patents Anti-Cancer Drug Discov 9:35–65
Gharwan H, Groninger H (2016) Kinase inhibitors and monoclonal antibodies in oncology: clinical implications. Nat Rev Clin Oncol 13:209–227
Hamblett KJ, Senter PD, Chace DF, Sun MMC, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody conjugate. Clin Cancer Res 10:7063–7070
Hamilton GS (2015) Antibody-drug conjugates for cancer therapy: the technological and regulatory challenges of developing drug-biologics hybrids. Biologicals 43:318–332
Han TH, Zhao B (2014) Absorption, distribution, metabolism, and excretion considerations for the development of antibody-drug conjugates. Drug Metab Dispos 42:1914–1920
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT (2010) The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9:325–338
Ho RJY, Chien J (2014) Trends in translational medicine and drug targeting and delivery: new insights on an old concept—targeted drug delivery with antibody-drug conjugates for cancers. J Pharm Sci 103:71–77
Hock MB, Thudium KE, Carrasco-Triguero M, Schwabe NF (2015) Immunogenicity of antibody drug conjugates: bioanalytical methods and monitoring strategy for a novel therapeutic modality. AAPS J 17:35–43
Hogarth PM, Pietersz GA (2012) Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov 11:311–331
Jackson D, Stover D (2015) Using the lessons learned from the clinic to improve the preclinical development of antibody drug conjugates. Pharm Res 32:3458–3469
Jain N, Smith SW, Ghone S, Tomczuk B (2015) Current ADC linker chemistry. Pharm Res 32:3526–3540
Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN (1992) Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res 52:5144–5153
Kamath AV, Iyer S (2016) Challenges and advances in the assessment of the disposition of antibody-drug conjugates. Biopharm Drug Dispos 37:66–74
Khawar IA, Kim JH, Kuh H-J (2015) Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release 201:78–89
Kim EG, Kim KM (2015) Strategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeutics. Biomol Ther 23(6):493–509
Kitson SL, Cuccurullo V, Moody TS, Mansi L (2013) Radionuclide antibody-conjugates, a targeted therapy towards cancer. Curr Radiopharm 6:57–71
Kline T, Steiner AR, Penta K, Sato AK, Hallam TJ, Yin G (2015) Methods to make homogeneous antibody conjugates. Pharm Res 32:3480–3493
Kraynov E, Kamath AV, Walles M, Tarcsa E, Deslandes A, Iyer RA, Datta-Mannan A, Sriraman P, Bairlein M, Yang JJ, Barfield M, Xiao G, Escandon E, Wang W, Rock DA, Chemuturi NV, Moore DJ (2016) Current approaches for absorption, distribution, metabolism, and excretion characterization of antibody-drug conjugates: an industry white paper. Drug Metab Dispos 44:617–623
Lambert JM (2012) Durg-conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol 76:248–262
Lammers T, Kiessling F, Hennink WE, Storm G (2012) Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release 161:175–187
Liu L (2015) Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci 104:1866–1884
Liu A, Kozhich A, Passmore D, Gu H, Wong R, Zambito F, Rangan VS, Myler H, Aubry A-F, Arnold ME, Wang J (2015) Quantitative bioanalysis of antibody-conjugated payload in monkey plasma using a hybrid immunocapture LC–MS/MS approach: assay development, validation, and a case study. J Chromatogr B 1002:54–62
Luo Q, Chung HH, Borths C, Janson M, Wen J, Joubert MK, Wypych J (2016) Structural characterization of a monoclonal antibody–maytansinoid immunoconjugate. Anal Chem 88:695–702
Mack F, Ritchie M, Sapra P (2014) The next generation of antibody drug conjugates. Semin Oncol 41(5):637–652
Maderna A, Leverett CA (2015) Recent advances in the development of new auristatins: structural modifications and application in antibody drug conjugates. Mol Pharm 12:1798–1812
Makuch RW, Shi R (2014) Comparison of drug approvals in Europe versus the United States: an analysis of discrepancies between drug products reviewed by EMA and FDA. Ther Innov Regul Sci 48:362–366
McCombs JR, Owen SC (2015) Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J 17:339–351
McDonagh CF, Turcott E, Westendorf L, Webster JB, Alley SC, Kim K, Andreyka J, Stone I, Hamblett KJ, Francisco JA, Carter P (2006) Engineered antibody-drug conjugate with defined sites and stoichiometries of drug attachment. Prot Eng Des Sel 19:299–307
Moussa EM, Panchal JP, Moorthy BS, Blum JS, Joubert MK, Narhi LO, Topp EM (2016) Immunogenicity of therapeutic protein aggregates. J Pharm Sci 105:417–430
Navarro-Teulon I, Lozza C, Pelegrin A, Vives E, Pouget J-P (2013) General overview of radioimmunotherapy of solid tumors. Immunotherapy 5:467–487
Nolting B (2013) Linker technologies for antibody conjugates. Method Mol Biol 1045:71–100
Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM (2014) Antibody-drug conjugates: current status and future directions. Drug Discov Today 19:869–881
Polakis P (2015) Antibody drug conjugates for cancer therapy. Pharmacol Rev 68:3–19
Prasad V (2014) The withdrawal of drugs for commercial reasons: the incomplete story of tositumomab. J Am Med Assoc Intern Med 174:1887–1888
Ross PL, Wolfe JL (2016) Physical and chemical stability of antibody drug conjugates: current status. J Pharm Sci 105:391–397
Schumacher D, Hackenberger CPR, Leonhardt H, Helma J (2016) Current status: site-specific antibody drug conjugates. J Clin Immunol. doi:10.1007/s10875-016-0265-6
Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12:278–287
Shefet-Carasso LR, Benhar I (2015) Antibody-targeted drugs and drug resistance-challenges and solutions. Drug Resist Updates 18:36–46
Sievers EL, Senter PD (2013) Antibody-drug conjugates in cancer therapy. Annu Rev Med 64:15–29
Singh SK, Luisi DL, Pak RH (2015) Antibody-drug conjugates: design, formulation, and physicochemical stability. Pharm Res 32:3541–3571
Sliwkowski MX, Mellman I (2013) Antibody therapeutics in cancer. Science 341:1192–1198
Strop P, Delaria K, Foletti D, Witt JM, Hasa-Moreno A, Poulsen K, Casas MG, Dorywalska M, Farias S, Pios A, Lui V, Dushin R, Zhou D, Navaratnam T, Tran T-T, Sutton J, Lindquist KC, Han B, Liu S-H, Shelton DL, Pons J, Rajpal A (2015) Site-specific conjugation improves therapeutic index of antibody drug conjugates with high drug loading. Nat Biotechnol 33:694–696
Tang L, Persky AM, Hochhaus G, Meibohm B (2004) Pharmacokinetic aspects of biotechnology products. J Pharm Sci 93:2184–2204
Torchilin VP (2014) Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 13:813–827
Van den Mooter T, Teuwen L-A, Rutten A, Dirix L (2015) Trastuzumab emtansine in advanced human epidermal growth factor receptor 2-positive breast cancer. Expert Opin Biol Ther 15:749–760
van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JGJ, Aalberse RC, Parren PWHI (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317:1554–1557
Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:1–17
Vugmeyster Y, Xu X, Theil F-P, Khawli LA, Leach MW (2012) Pharmacokinetics and toxicology of therapeutic proteins: advances and challenges. World J Biol Chem 3:73–92
Wakankar A, Chen Y, Gokarn Y, Jacobson FS (2011) Analytical methods for physicochemical characterization of antibody drug conjugates. MAbs 3:161–172
Wong DJL, Hurvitz SA (2014) Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates. Ann Transl Med 2:122
Acknowledgments
Helpful discussions with Dr. James Chovan are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakhtiar, R. Antibody drug conjugates. Biotechnol Lett 38, 1655–1664 (2016). https://doi.org/10.1007/s10529-016-2160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2160-x