Abstract
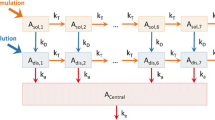
The convolution-based modeling approach has been shown to be flexible and easy to implement for performing a deconvolution analysis and for assessing in vitro/in vivo correlation using non-linear regression and a pre-specified model describing the in vivo drug absorption. A generalization of this method has been developed using a nonparametric description of the in vivo drug absorption process in replacement of a model-based definition. A comparison of the parametric and nonparametric deconvolution and convolution analyses was conducted on the pharmacokinetic (PK) data observed in four published studies after the administration of an extended-release formulation of methylphenidate at the dose of 18 mg. All the analyses were conducted using a conventional non-linear regression software (NONMEM). The results of the deconvolution analysis indicated that the parametric and nonparametric approaches performed similarly. The parametric approach described the input function using a double Weibull equation (6 parameters) while the nonparametric approach described the input function using a piecewise approximation (12–13 parameters). The validation of the results of the deconvolution analysis was conducted by comparing observed and predicted PK concentrations by the convolution analysis. The performance of the parametric and nonparametric approaches for assessing deconvolution was evaluated using the Akaike and the Bayesian information criteria. These criteria indicated that, despite the similar results obtained with the two approaches, the nonparametric approach provided better results. In conclusion, these results indicated that the nonparametric approach should be considered as the preferred approach for conducting a deconvolution analysis.



Similar content being viewed by others
References
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: extended release oral dosage forms: development, evaluation, and application of in-vitro/in-vivo correlations. 1997. http://www.fda.gov/downloads/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070239.pdf. Accessed 25 Aug 2019.
Gomeni R, Fang LL, Bressolle-Gomeni F, Spencer TJ, Faraone SV, Babiskin A. A general framework for assessing in-vitro/in-vivo correlation as a tool for maximizing the benefit-risk ratio of a treatment using a convolution-based modeling approach. CPT Pharmacometrics Syst Pharmacol. 2019;8(2):97–106.
Wagner JG, Nelson E. Per cent absorbed time plots derived from blood level and/or urinary excretion data. J Pharm Sci. 1963;52(6):610–1.
Loo JCK, Riegelman S. New method for calculating the intrinsic absorption rate of drugs. J Pharm Sci. 1968;57(6):918–28.
Cutler DJ. Numerical deconvolution by least squares: use of prescribed input functions. J Pharmacokinet Biopharm. 1978;6(3):227–41.
Veng-Pedersen P. Linear and Nonlinear system approaches in pharmacokinetics: how much do they have to offer? I. General considerations. J Pharmacokinet Biopharm. 1988;16(5):413–32.
Madden FN, Godfrey KR, Chappell MJ, Hovorka R, Bates RA. A comparison of six deconvolution techniques. J Pharmacokinet Biopharm. 1996;24(3):283–99.
Verotta D. Concepts, properties and applications of linear systems to describe distribution, identify input, and control endogenous substances and drugs in biological systems. Crit Rev Biomed Eng. 1996;24(2–3):73–139.
Pitsiu M, Sathyan G, Gupta S, Verotta D. A semiparametric deconvolution model to establish in-vivo-in-vitro correlation applied to OROS oxybutynin. J Pharm Sci. 2001;90(6):702–12.
Buchwald P. Direct differential-equation-based in-vitro-in-vivo correlation (IVIVC) method. J Pharm Pharmacol. 2003;55(4):495–504.
Gaynor C, Dunne A, Costello C, Davis J. A population approach to in-vitro-in-vivo correlation modelling for compounds with nonlinear kinetics. J Pharmacokinet Pharmacodyn. 2011;38(3):317–32.
Rescigno A. Compartmental analysis and its manifold applications to pharmacokinetics. AAPS J. 2010;12(1):61–72.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Population Pharmacokinetics Guidance for Industry, Draft guidance 2019. https://www.fda.gov/media/128793/download. Accessed 25 Aug 2019.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2(4):e38.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Draft guidance on methylphenidate hydrochloride. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/psg/Methylphenidate%20Hydrochloride_draft_Oral%20tab%20ER_RLD%2021121_RC07-18.pdf. Accessed 27 Oct 2019.
Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–36.
González MA, Pentikis HS, Anderl N, Benedict MF, DeCory HH, Dirksen SJ, et al. Methylphenidate bioavailability from two extended-release formulations. Int J Clin Pharmacol Ther. 2002;40(4):175–84.
Modi NB, Wang B, Hu WT, Gupta SK. Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects. Biopharm. Drug Dispos. 2000;21(1):23–31.
Schapperer E, Daumann H, Lamouche S, Thyroff-Friesinger U, Viel F, Weitschies W. Bioequivalence of Sandoz methylphenidate osmotic controlled release tablet with Concerta® (Janssen-Cilag). Pharmacol Res Perspect. 2015;3(1):e00072.
Kimko H, Gibiansky E, Gibiansky L, Starr HL, Berwaerts J, Massarella J, et al. Population pharmacodynamic modeling of various extended-release formulations of methylphenidate in children with attention deficit hyperactivity disorder via meta-analysis. J Pharmacokinet Pharmacodyn. 2012;39(2):161–76.
Gomeni R, Bressolle-Gomeni F, Spencer TJ, Faraone SV, Fang L, Babiskin A. Model-based approach for optimizing study design and clinical drug performances of extended-release formulations of methylphenidate for the treatment of ADHD. Clin Pharmacol Ther. 2017;102(6):951–60.
Adjei A, Kupper RJ, Teuscher NS, Wigal S, Sallee F, Childress A, et al. Steady-state bioavailability of extended-release methylphenidate (MPH-MLR) capsule vs. immediate-release methylphenidate tablets in healthy adult volunteers. Clin Drug Investig. 2014;34(11):795–805.
Kesisoglou F, Balakrishnan A, Manser K. Utility of PBPK Absorption modeling to guide modified release formulation development of gaboxadol, a highly soluble compound with region-dependent absorption. J Pharm Sci. 2016;105(2):722–8.
Stillhart C, Pepin X, Tistaert C, Good D, Van Den Bergh A, Parrott N, et al. PBPK Absorption modeling: establishing the in-vitro-in-vivo link-industry perspective. AAPS J. 2019;21(2):19.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Gomeni, R., Bressolle-Gomeni, F. Deconvolution Analysis by Non-linear Regression Using a Convolution-Based Model: Comparison of Nonparametric and Parametric Approaches. AAPS J 22, 9 (2020). https://doi.org/10.1208/s12248-019-0389-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0389-8